References
[1] https://www.zailaboratory.com/zai-lab-to-present-data-from-phase-1-trial-of-zl-1310-its-investigational-antibody-drug-conjugate-adc-for-dll3-solid-tumors-at-eortc-nci-aacr-symposium-ena-2024-18/
[2] https://www.nature.com/articles/d41573-024-00088-2
[3] Giffin, Michael J., et al. "AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer." Clinical Cancer Research 27.5 (2021): 1526-1537.
[4] Bulman, Michael P., et al. "Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis." Nature genetics 24.4 (2000): 438-441.
[5] Steinbuck, Martin Peter, and Susan Winandy. "A review of notch processing with new insights into ligand-independent notch signaling in T-cells." Frontiers in immunology 9 (2018): 1230.
[6] Hu, Bingxin, et al. "Over-expression of human Notch ligand Delta-like 3 promotes proliferation of human gastric cancer cells in vitro." Nan Fang yi ke da xue xue bao= Journal of Southern Medical University 38.1 (2018): 14-19.
[7] Saunders, Laura R., et al. "A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo." Science translational medicine 7.302 (2015): 302ra136-302ra136.
[8] Stanley, Pamela. "Regulation of Notch signaling by glycosylation." Current opinion in structural biology 17.5 (2007): 530-535.
[9] Chapman, Gavin, et al. "Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis." Human molecular genetics 20.5 (2011): 905-916.
[10] Deng, San-Ming, et al. "The Notch ligand delta-like 3 promotes tumor growth and inhibits Notch signaling in lung cancer cells in mice." Biochemical and biophysical research communications 483.1 (2017): 488-494.
[11] Venkatesh, Deepak, et al. "RhoA-mediated signaling in Notch-induced senescence-like growth arrest and endothelial barrier dysfunction." Arteriosclerosis, thrombosis, and vascular biology 31.4 (2011): 876-882.
[12] Wang, Jianpeng, et al. "EGFL7 participates in regulating biological behavior of growth hormone–secreting pituitary adenomas via Notch2/DLL3 signaling pathway." Tumor Biology 39.7 (2017): 1010428317706203.
[13] Ding, Xiaojie, Fuyao Li, and Li Zhang. "Knockdown of Delta-like 3 restricts lipopolysaccharide-induced inflammation, migration and invasion of A2058 melanoma cells via blocking Twist1-mediated epithelial-mesenchymal transition." Life sciences 226 (2019): 149-155.
[14] Jia, Dongyu, et al. "NOTCH2/NOTCH3/DLL3/MAML1/ADAM17 signaling network is associated with ovarian cancer." Oncology letters 17.6 (2019): 4914-4920.
[15] Huang, Jianling, et al. "DLL3 is regulated by LIN28B and miR-518d-5p and regulates cell proliferation, migration and chemotherapy response in advanced small cell lung cancer." Biochemical and biophysical research communications 514.3 (2019): 853-860.
[16] Sabari, Joshua K., et al. "Unravelling the biology of SCLC: implications for therapy." Nature reviews Clinical oncology 14.9 (2017): 549-561.
[17] Vitorino, Philip, et al. "Rova-T enhances the anti-tumor activity of anti-PD1 in a murine model of small cell lung cancer with endogenous Dll3 expression." Translational oncology 14.1 (2021): 100883.
[18] Ayyanan, Ayyakannu, et al. "Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism." Proceedings of the National Academy of Sciences 103.10 (2006): 3799-3804.
[19] Yan, S., et al. "Expression profile of Notch‐related genes in multidrug resistant K562/A02 cells compared with parental K562 cells." International journal of laboratory hematology 32.2 (2010): 150-158.
[20] Maemura, Kentaro, et al. "Delta-like 3 is silenced by methylation and induces apoptosis in human hepatocellular carcinoma." International journal of oncology 42.3 (2013): 817-822.
[21] Turchi, Laurent, et al. "Tumorigenic potential of miR‐18A* in glioma initiating cells requires NOTCH‐1 signaling." Stem Cells 31.7 (2013): 1252-1265.
[22] Jungk, Christine, et al. "Spatial transcriptome analysis reveals Notch pathway-associated prognostic markers in IDH1 wild-type glioblastoma involving the subventricular zone." BMC medicine 14.1 (2016): 1-16.
[23] Bauer, Todd M., et al. "ORAL02. 01: safety and efficacy of single-agent rovalpituzumab tesirine, a DLL3-targeted ADC, in recurrent or refractory SCLC: topic: medical oncology." Journal of Thoracic Oncology 11.11 (2016): S252-S253.
[24] Hamamoto, Hiroki, et al. "Delta-like 3 is silenced by HBx via histone acetylation in HBV-associated HCCs." Scientific reports 8.1 (2018): 1-11.
[25] Spino, Marissa, et al. "Cell surface Notch ligand DLL3 is a therapeutic target in isocitrate dehydrogenase–mutant glioma." Clinical Cancer Research 25.4 (2019): 1261-1271.
[26] Mizoguchi, Masahiro, et al. "Molecular characteristics of glioblastoma with 1p/19q co-deletion." Brain tumor pathology 29.3 (2012): 148-153.
[27] Song, Hai‑Yan, et al. "Expression of Notch receptors and their ligands in pancreatic ductal adenocarcinoma." Experimental and therapeutic medicine 16.1 (2018): 53-60.
[28] Kiniwa, Yukiko, et al. "Delta-like protein 3 promotes proliferation and migration of melanoma via MAPK activation." Journal of Dermatological Science 84.1 (2016): e176.
[29] Nakahara, Satoshi, et al. "AT-rich interaction domain-containing protein 3B has a potential to be a new stem cell marker of melanoma." Journal of Dermatological Science 84.1 (2016): e176-e177.
[30] Wang, Juan, et al. "Upregulated delta-like protein 3 expression is a diagnostic and prognostic marker in endometrial cancer: a retrospective study." Medicine 97.51 (2018).

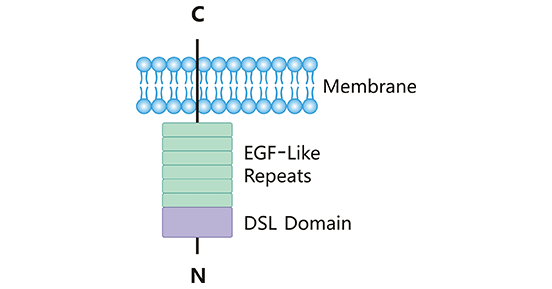
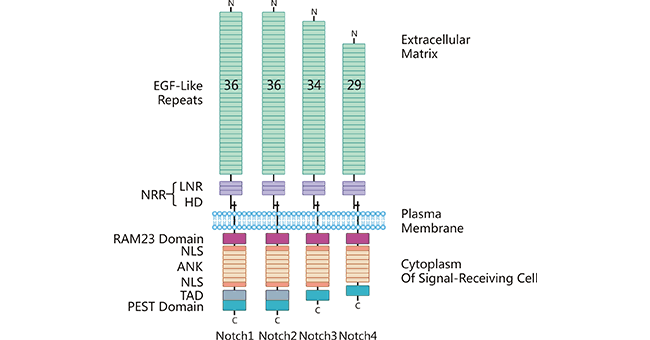
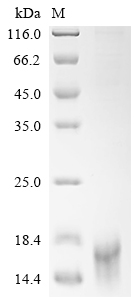
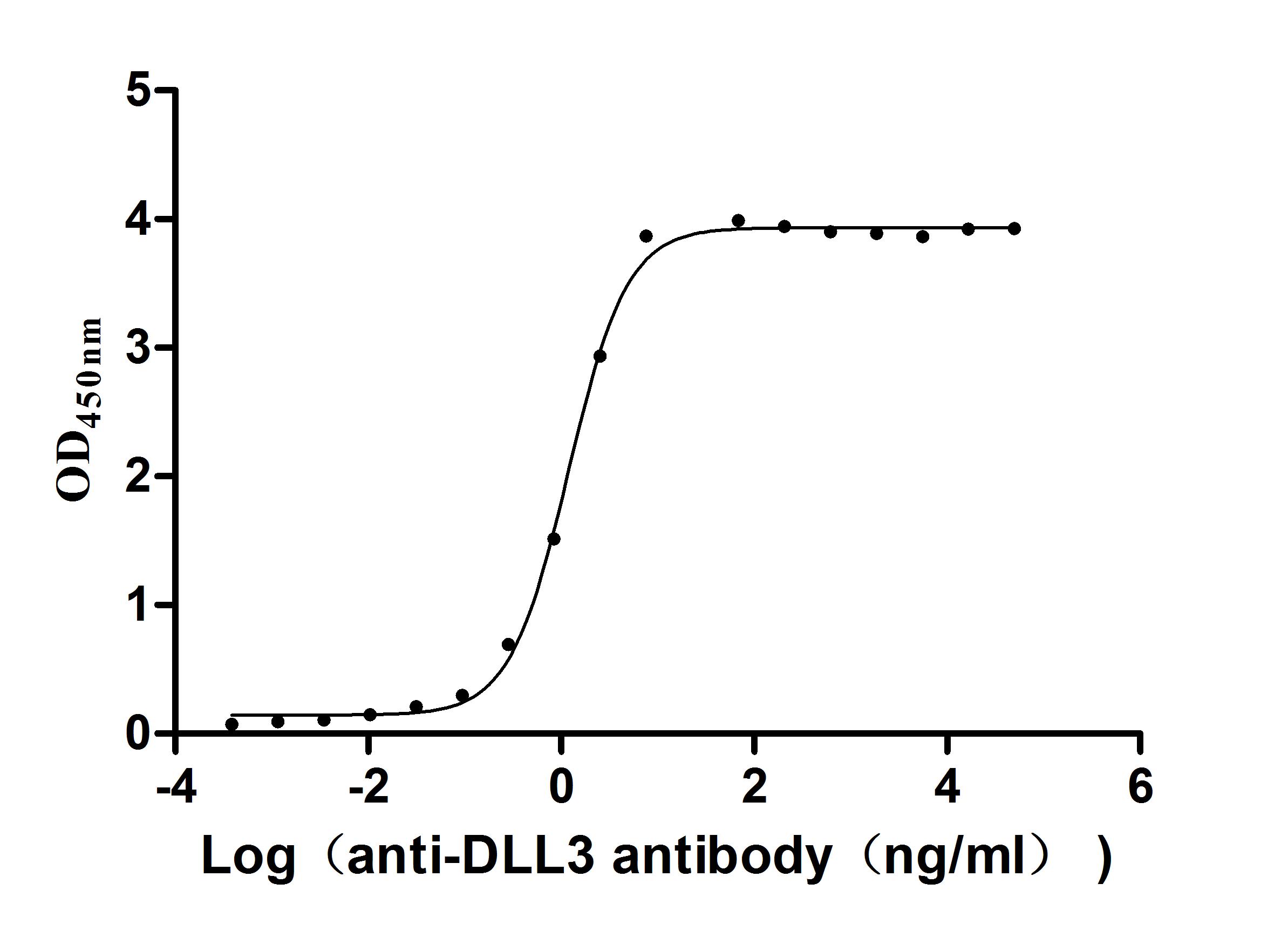
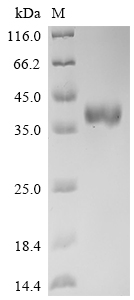
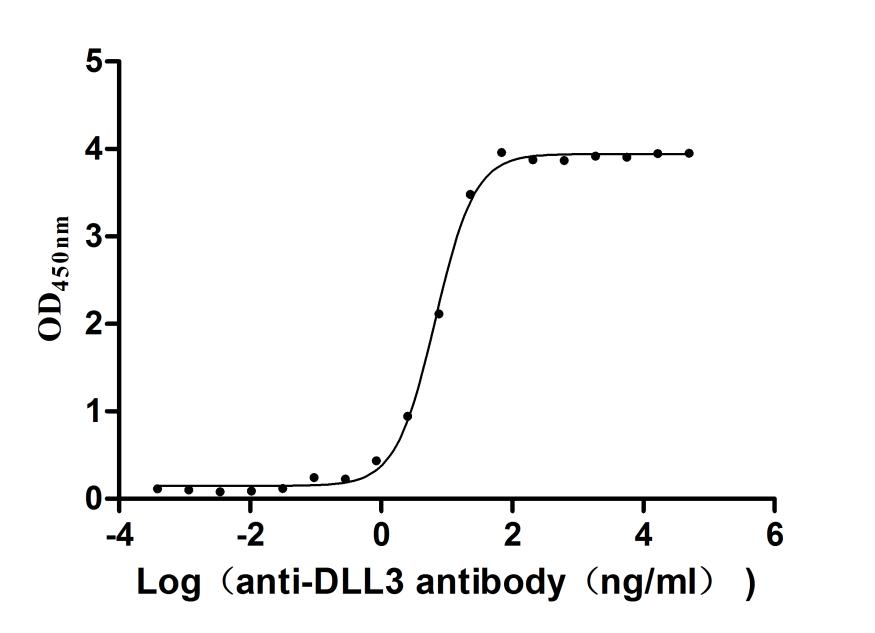
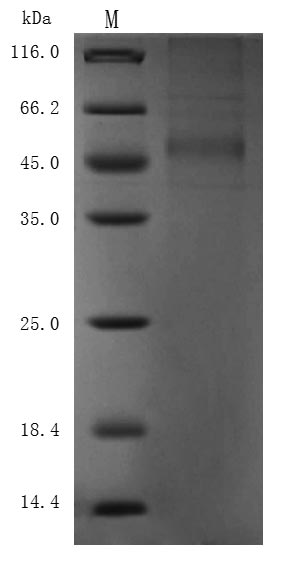
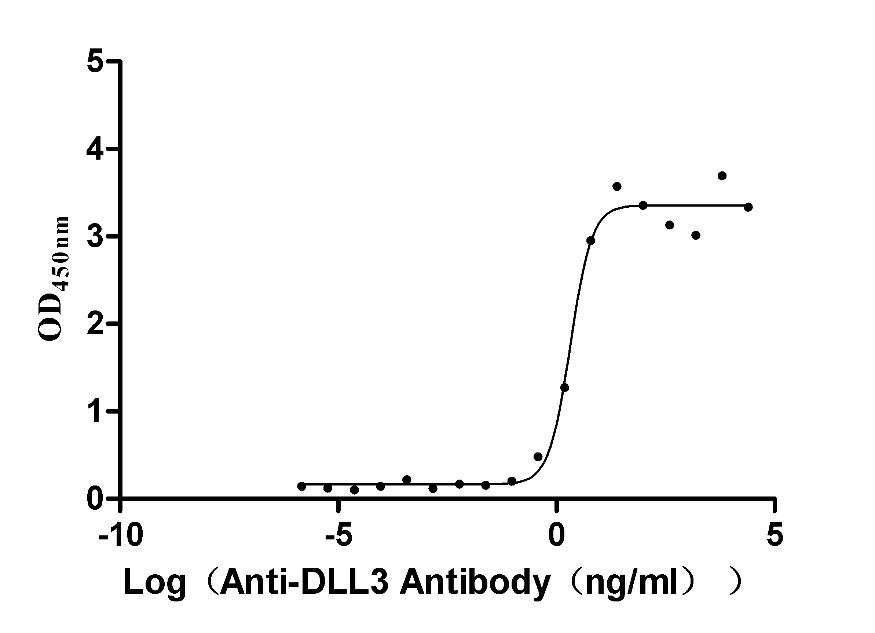



Comments
Leave a Comment