On April 19, 2022, Innovative Cellular Therapeutics (ICT) company from Shanghai, China receives FDA Fast Track Designation for GCC19CART. GCC19CART is a CAR-T cell therapy based on ICT's unique CoupledCAR® technology platform that targets GUCY2C to treat relapsed refractory metastatic colorectal cancer (R/R mCRC) [1]. Moreover, ICT plans to initiate a phase I clinical trial for GCC19CART in the U.S. in mid-2022. In fact, this follows previous positive results from a proof-of-concept (PoC) human trial of GCC19CART in China. Apparently, it is well document that GUCY2C holds a huge potential in tumor immunotherapy, especially for CRC CAR T therapy. But what is GUCY2C? How’s the progress of GUCY2C in colorectal cancer immunotherapy? Let's find out in today's article that explores the GUCY2C' functions and its potentials in immunotherapy.
1. What is the Structure and Function of GUCY2C?
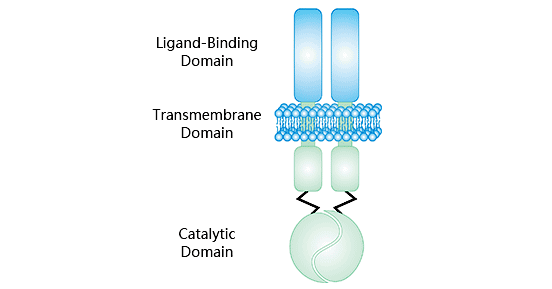
Guanylate cyclase C (GUCY2C/GCC) belongs to the family of receptor guanylate cyclases. GUCY2 as an intestinal receptor for bacterial heat-stable enterotoxins, it is also known as the heat-stable enterotoxin receptor [2-4]. The human GUCY2C gene is located on chromosome 12q12, encoding a type I transmembrane protein with a molecular mass approximately 120 kDa [5]. As shown in Figure 1, the GUCY2C protein structure consists of five main parts: (i) the extracellular ligand binding domain; (ii) the hydrophobic transmembrane domain, which converts an extracellular signal into the intracellular fluid; (iii) the cytoplasmic domain, which transmits signals to the catalytic region; (iv) the catalytic region; (v) the carboxyl-terminal region [6, 7]. Numerous studies have shown that the activation of GUCY2C stimulates the production of cyclic guanosine monophosphate (cGMP), an intracellular second messenger, which plays a role in regulating homeostasis, maintaining intestinal barrier function, and exerting anti-inflammatory effects in vivo [8].
Figure 1. GUCY2C structure
In normal human tissues, GUCY2C is mainly expressed in intestinal epithelial cells, but not in extraintestinal tissues and tumors. GUCY2C is involved in signal transduction pathways that play important roles in the maintenance of fluid and electrolyte balance [6]. Recent studies have revealed that GUCY2C is stably expressed in primary colorectal cancer cells, while in metastatic colorectal cancer cells, GUCY2C is abnormally highly expressed and is considered as a specific marker molecule for metastatic colorectal cancer [9]. Additional data showed that GUCY2C was expressed in pancreatic, gastric and esophageal cancer [10], suggesting that GUCY2C may be a potential target for these diseases.
2. What are the Ligands of the GUCY2C?
The ligands of the GUCY2C include an exogenous ligand, heat-stable enterotoxin (STa), of bacterial origin, and the two endogenous peptides, guanylin (Gn) and uroguanylin (Ugn). Gn and Ugn are often expressed in many human tissues and organs, such as the intestine, kidney, heart, lung, uterus, and ovary, with particularly abundant expression in the intestine and kidney [11].
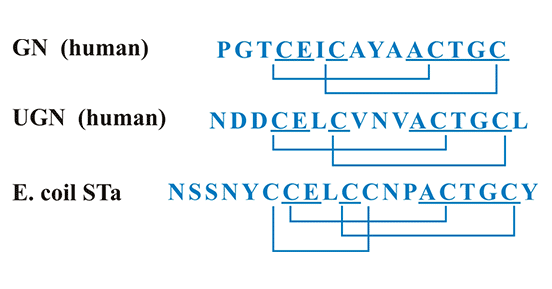
Gn and Ugn are structurally similar to bacterial STa, all which contain abundant cysteine and disulfide bonds in their peptide chains (Figure 2) [7]. This structural feature plays an essential role in biological functions. The number of disulfide bonds divides the ligands of GUCY2C into two categories: Gn and Ugn with three disulfide bonds; STa with two disulfide bonds. The difference in the number of cysteines and disulfide bonds determines the differences in their biological functions [13]. It has been suggested that Gn, Ugn, and STa all inhibit the absorption of fluid and salt in the intestine, but with different inhibitory abilities [13]. GUCY2C could be activated as these ligands (Gn/Ugn/STa) bind to the GUCY2C in intestinal epithelial cells. Moreover, the differences in protein sequence and conformation of Gn, Ugn and STa lead to their different affinities for binding to the GUCY2C and the different abilities to activate the GUCY2C [13].
Figure 2. Amino acid sequences of human Gn, Ugn, and STa
3. How Does the GUCY2C-Mediated Signal Transduction Pathway?
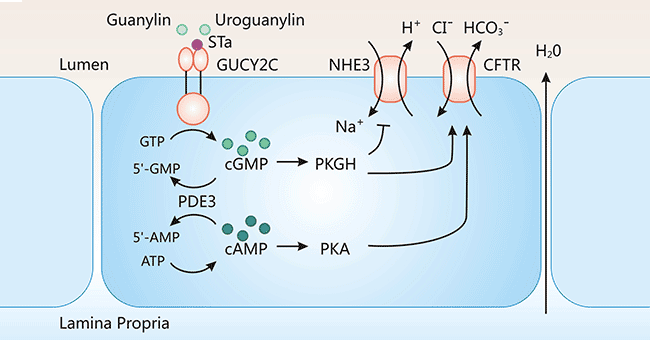
Current studies indicated that GUCY2C is usually involved in the opening of ion channels in the cell membrane. GUCY2C functions as a regulator to maintain normal intra- and extracellular ion concentrations together with fluid and electrolyte balance. Besides, GUCY2C participates into facilitating intestinal digestion and absorption, maintaining intestinal function, and protecting the intestinal barrier integrity. As indicated in Figure 3, GUCY2C activation upon binding to the ligand (Gn/Ugn/STa) mediates the conversion of GTP to cGMP. Furthermore, the increase of intracellular concentration of cGMP contributes to the activation of downstream effectors, including cGMP-dependent protein kinases PKGs, phosphodiesterases PDEs, and cyclic nucleotide-gated ions (CNG). The activation of a series of signaling molecules is closely associated with human metabolic mechanisms. For example, when GUCY2C-induced protein kinase G II (PKG II) is activated, it phosphorylates cystic fibrosis transmembrane conductance regulator (CFTR). Upon CFTR phosphorylation, it induces the movement of chloride ions (Cl−) and others from intracellular to extracellular fluids. When GUCY2C ligand is absent, the abnormal expression of GUCY2C will lead to an homeostasis imbalance in colorectal epithelial cells, which can cause disruption of the intestinal barrier and promote intestinal tumorigenesis, but the specific regulatory mechanism needs to be further elucidated [14].
Figure 3. GUCY2C-mediated signal transduction pathway
4. The Role of GUCY2C in the Diseases
GUCY2C is activated by guanylin, uroguanylin, and heat-stable enterotoxin, which could regulate epithelial cell dynamics and the homeostatic balance of proliferation, metabolism, and differentiation. Current studies identified that GUCY2C is mainly associated with intestinal diseases, as well as less commonly reported neurological diseases. Among them, the intestinal related diseases commonly includes functional gastrointestinal diseases (e.g. allergic bowel syndrome, constipation, or acidity overload), inflammatory bowel diseases (e.g. Crohn's disease and ulcerative colitis, etc.), and cancer (e.g. gastrointestinal cancers).
It has been suggested that mutations in activated GUCY2C can cause changes in the intestinal environment, which can lead to altered intestinal flora and increased susceptibility to Crohn's disease [15]. In addition, GUCY2C is associated with congenital sodium diarrhea and familial GUCY2C diarrhea syndrome can develop into inflammatory bowel disease in adulthood [16]. In terms of neurological disorders, a study suggested that GUCY2C is selectively expressed on midbrain dopamine neurons and regulates attention and activity levels in animals by unique physiological mechanisms [17].
More importantly, GUCY2C has a strong positive expression in the peripheral blood of patients with colorectal cancer, suggesting that GUCY2C can be used as an early detection indicator of postoperative recurrence and metastasis in patients with colorectal cancer [18]. Another study showed that GUCY2C was highly expressed in gastric intestinal metaplasia, atypical hyperplasia, and gastric cancer [19]. It has also been reported that GUCY2C is a more specific marker to distinguish primary and metastatic ovarian mucinous tumors [20]. Of interest, a large number of studies suggested that GUCY2C is often abnormally expressed in the early stages of colon carcinogenesis [21, 22]. Some studies have found that GUCY2C is specifically highly expressed in metastatic colorectal cancer cells. In a nutshell, it is implied that GUCY2C might act as a potential molecular marker for colorectal cancer.
5. GUCY2C Targeted Therapy and Clinical Perspectives
GUCY2C as an emerging target in immunotherapy, the FDA has granted Fast Track Designation to GCC19CART based on its potential to address the unmet need for patients with R/R mCR. Early days, clinical data from one research site for GCC19CART on AACR meeting just had been revealed, which shows the well efficient in patients. Beyond this, GUCY2C has been studied in other immunotherapies such as bispecific antibody, vaccines, and immunotoxins. Aforementioned preclinical studies from Pfizer's indicated that GUCY2C-CD3 bispecific antibody PF-0706119 has good anti-tumor efficacy [23]. At present, preclinical studies of PF-07062119 are still ongoing and its efficacy needs to be further validated in clinical trials. In addition, the Sidney Kimmel Cancer Center of the Thomas Jefferson University has developed a vaccine specifically for colorectal cancer, Ad5-GUCY2C-PADRE, which links the GUCY2C to PADRE to enhance immune responses. Preclinical studies have shown that this vaccine activates CD8+ T cells to produce an immune response and prevent colorectal cancer metastasis [24].
GUCY2C is an important member of the intestinal signaling system. Colorectal cancer, as the third most common cancer worldwide, the recent GUCY2C targeted research has highlighted the important role of GUCY2C in colorectal cancer. Moreover, the application of GUCY2C CAR-T or vaccine to colorectal cancer treatment is a major breakthrough. The progress in the development of new immunotherapies will certainly bring new insights to the treatment of gastrointestinal malignancies, particularly for colorectal cancer.
To fully serve the pharmaceutical companies in GUCY2C based drug research for colorectal cancer (CRC) treatment. CUSABIO has launched the GUCY2C active protein product (Code: CSB-MP010053HUd9; Code: CSB-MP010053HU) to help you explore the mechanism of GPRC5D or its potential clinical value.
● Recombinant Human Heat-stable enterotoxin receptor(GUCY2C),partial (Active)
High Purity Validated by Western Blot (WB)
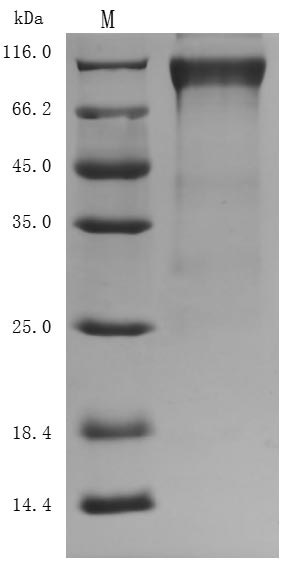
The purity was greater than 94.8% as determined by SDS-PAGE. (Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
Excellent Bioactivity Validated by Functional ELISA
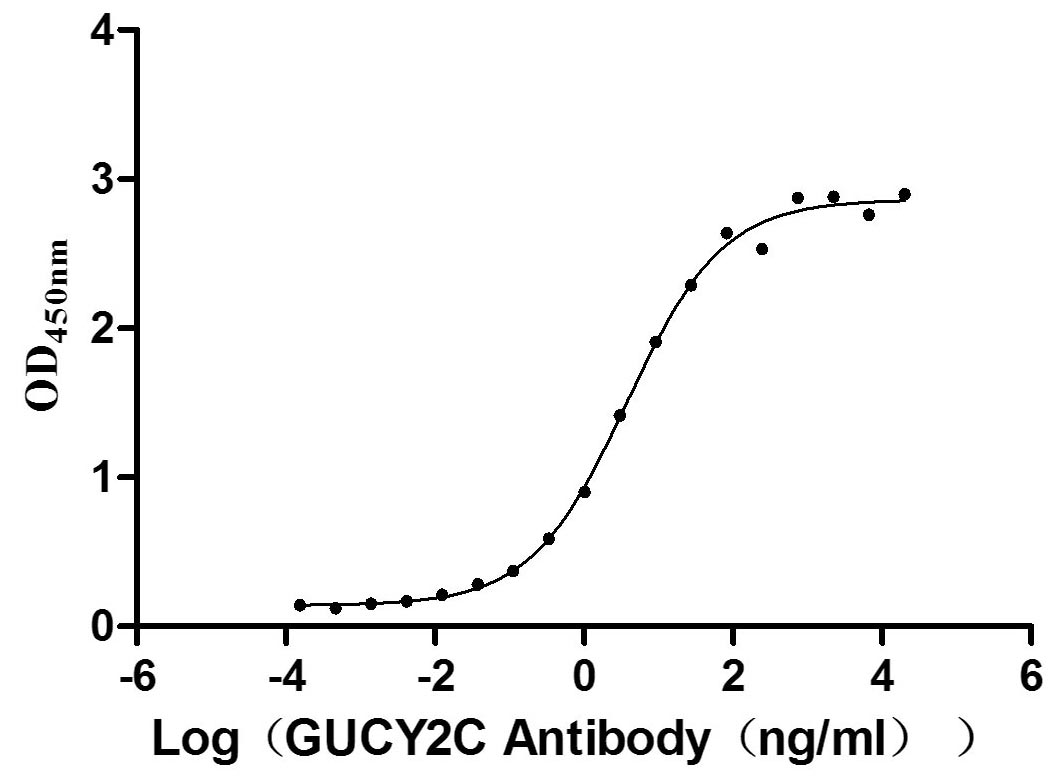
Immobilized human GUCY2C at 5 μg/ml can bind Anti-GUCY2C recombinant antibody (CSB-RA010053A2HU), the EC50 is 3.049-4.660 ng/mL.
● Recombinant Human Heat-stable enterotoxin receptor(GUCY2C),partial (Active)
High Purity Validated by Western Blot (WB)
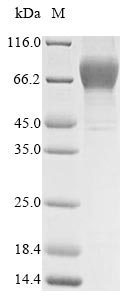
The purity was greater than 95% as determined by SDS-PAGE. (Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
Excellent Bioactivity Validated by Functional ELISA
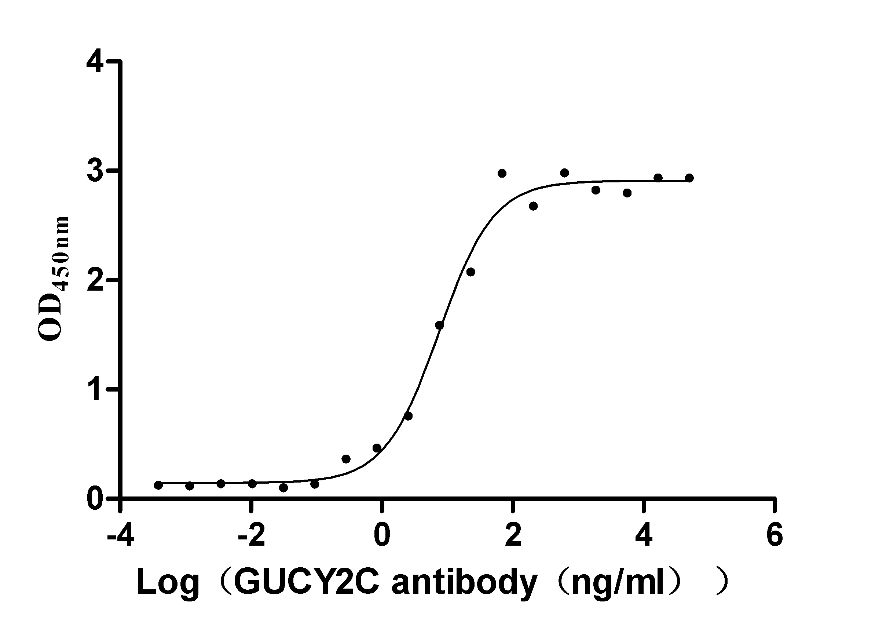
Immobilized human GUCY2C at 5 μg/ml can bind Anti-GUCY2C recombinant antibody (CSB-RA010053A2HU), the EC50 is 11.89-24.13 ng/mL.
References
[1] Chen, Naifei, et al. "Dose Escalation Study of GCC19CART CoupledCAR® Technology for Patients with Relapsed or Refractory Colorectal Cancer." Blood 138.Supplement 1 (2021): 4841-4841.
[2] Lisby, Amanda N., et al. "GUCY2C as a biomarker to target precision therapies for patients with colorectal cancer." Expert Review of Precision Medicine and Drug Development 6.2 (2021): 117-129.
[3] Snook, Adam. "GUCY2C‐Directed CAR‐T Cell Therapy for Upper‐GI Cancers." The FASEB Journal 35 (2021).
[4] Pattison, Amanda M., et al. "Silencing the intestinal GUCY2C tumor suppressor axis requires APC loss of heterozygosity." Cancer biology & therapy 21.9 (2020): 799-805.
[5] Flickinger Jr, John C., et al. "Guanylyl cyclase C as a biomarker for immunotherapies for the treatment of gastrointestinal malignancies." Biomarkers in Medicine 15.3 (2021): 201-217.
[6] Hasegawa M, Hidaka Y, Matsumoto Y, Sanni T, Shimonishi Y. Determination of the binding site on the extracellular domain of guanylyl cyclase C to heat-stable enterotoxin. J Biol Chem. 1999 Oct 29;274(44):31713-8.
[7] Potter, Lincoln R. "Domain analysis of human transmembrane guanylyl cyclase receptors: implications for regulation." Front Biosci 10.1 (2005): 1205-1220.
[8] Blomain, Erik S., et al. "APC-β-catenin-TCF signaling silences the intestinal guanylin-GUCY2C tumor suppressor axis." Cancer biology & therapy 21.5 (2020): 441-451.
[9] Jimenez-Luna, Cristina, et al. "Circulating PTGS2, JAG1, GUCY2C and PGF mRNA in Peripheral Blood and Serum as Potential Biomarkers for Patients with Metastatic Colon Cancer." Journal of clinical medicine 10.11 (2021): 2248.
[10] Wilson, Chantell, et al. "The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer." Cancer Epidemiology and Prevention Biomarkers 23.11 (2014): 2328-2337.
[11] Romi, Hila, et al. "Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C." The American Journal of Human Genetics 90.5 (2012): 893-899.
[12] Lisby, Amanda N., et al. "GUCY2C as a biomarker to target precision therapies for patients with colorectal cancer." Expert Review of Precision Medicine and Drug Development 6.2 (2021): 117-129.
[13] GU, Xuemei, Lei FENG, and Maode LAI. "Research progress in guanylin family." Chinese Journal of Pathophysiology (2000).
[14] Rappaport, Jeffrey A., and Scott A. Waldman. "The guanylate cyclase C—cGMP signaling axis opposes intestinal epithelial injury and neoplasia." Frontiers in oncology 8 (2018): 299.
[15] Tronstad, Rune R., et al. "Guanylate cyclase C activation shapes the intestinal microbiota in patients with familial diarrhea and increased susceptibility for Crohn's disease." Inflammatory bowel diseases 23.10 (2017): 1752-1761.
[16] Nambu, Ryusuke, et al. "A Systematic Review of Monogenic Inflammatory Bowel Disease." Clinical Gastroenterology and Hepatology (2021).
[17] Liu, Lu, et al. "Association between GUC2C and ADHD: Evidence from both categorical and quantitative traits." Psychiatry research 220.1-2 (2014): 708-710.
[18] Bashir, Babar, et al. "Silencing the GUCA2A-GUCY2C tumor suppressor axis in CIN, serrated, and MSI colorectal neoplasia." Human pathology 87 (2019): 103-114.
[19] Snook, Adam E., Michael S. Magee, and Scott A. Waldman. "GUCY2C-targeted cancer immunotherapy: past, present and future." Immunologic research 51.2 (2011): 161-169.
[20] Ciocca, Vincenzo, et al. "Guanylyl cyclase C is a specific marker for differentiating primary and metastatic ovarian mucinous neoplasms." Histopathology 55.2 (2009): 182-188.
[21] Waldman, Scott A., and Michael Camilleri. "Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders." Gut 67.8 (2018): 1543-1552.
[22] Aka, Allison A., et al. "Guanylate cyclase C as a target for prevention, detection, and therapy in colorectal cancer." Expert review of clinical pharmacology 10.5 (2017): 549-557.
[23] Maresca, Kevin P., et al. "Preclinical Evaluation of 89Zr-Df-IAB22M2C PET as an Imaging Biomarker for the Development of the GUCY2C-CD3 Bispecific PF-07062119 as a T Cell Engaging Therapy." Molecular Imaging and Biology 23.6 (2021): 941-951.
[24] Snook, Adam E., et al. "Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients." Journal for immunotherapy of cancer 7.1 (2019): 1-12.
CUSABIO team. GUCY2C: a Novel Target in Tumor Immunotherapy, Specially in Colorectal Cancer CAR T Therapy!. https://www.cusabio.com/c-21069.html








Comments
Leave a Comment