[1] Malm-Erjefält, Monika, et al. "Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. "Drug Metabolism and Disposition 38.11 (2010): 1944-1953.
[2] Fujita, Hiroki, et al. "The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential." Kidney international 85.3 (2014): 579-589.
[3] Pabreja, Kavita, et al. "Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation ." British Journal of Pharmacology 171.5 (2014): 1114-1128.
[4] Varin, Elodie M., et al. "Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action." Cell reports 27.11 (2019): 3371-3384.
[5] Pyke, Charles, et al. "GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody." Endocrinology 155.4 (2014): 1280-1290.
[6] Zhou, Da, et al. "Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression." Experimental & molecular medicine 50.12 (2018): 1-12.
[7] Morrow, Nadya M., Antonio A. Hanson, and Erin E. Mulvihill. "Distinct identity of GLP-1R, GLP-2R, and GIPR expressing cells and signaling circuits within the gastrointestinal tract." Frontiers in Cell and Developmental Biology 9 (2021): 703966.
[8] Ejarque, Miriam, et al. "Role of adipose tissue GLP-1R expression in metabolic improvement after bariatric surgery in patients with type 2 diabetes." Scientific reports 9.1 (2019): 6274.
[9] Zhang, Xiaolong, et al. "Discovery of novel OXM-based glucagon-like peptide 1 (GLP-1)/glucagon receptor dual agonists." Peptides 161 (2023): 170948.
[10] Liu, Chunxia, Yuxing Zou, and Hai Qian. "GLP-1R agonists for the treatment of obesity: a patent review (2015-present)." Expert Opinion on Therapeutic Patents 30.10 (2020): 781-794.
[11] Cai, Xingguang, et al. "Novel glucagon-and OXM-based peptides acting through glucagon and GLP-1 receptors with body weight reduction and anti diabetic properties." Bioorganic Chemistry 95 (2020): 103538.
[12] HU, Zhong-ping, et al. "Research Progress on Structure and Function of GLP-1R and Screening for Small Molecule Drugs." Biotechnology Bulletin 33.2 ( 2017): 30.
[13] Meurot, C., et al. "Targeting the GLP-1/GLP-1R axis to treat osteoarthritis: a new opportunity?" Journal of Orthopaedic Translation 32 (2022): 121-129.
[14] Liang, Yi-Lynn, et al. "Dominant negative G proteins enhance formation and purification of agonist-GPCR-G protein complexes for structure determination." acs pharmacology & translational science 1.1 (2018): 12-20.
[15] Bitsi, Stavroula, et al. "Divergent acute versus prolonged pharmacological GLP-1R responses in adult β cell-specific β-arrestin 2 knockout mice." Science Advances 9.18 (2023): eadf7737.
[16] Challa, Tenagne Delessa, et al. "Regulation of adipocyte formation by GLP-1/GLP-1R signaling." Journal of Biological Chemistry 287.9 (2012): 6421- 6430.
[17] Broide, E., et al. "Reduced GLP-1R expression in gastric glands of patients with type 2 diabetes mellitus." The Journal of Clinical Endocrinology & Metabolism 99.9 (2014): E1691-E1695.
[18] Simó, Rafael, and Cristina Hernández. "GLP-1R as a target for the treatment of diabetic retinopathy: friend or foe?" Diabetes 66.6 (2017): 1453-1460.
[19] Meier, Juris J. "GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus." Nature Reviews Endocrinology 8.12 (2012): 728-742 .
[20] Bendotti, Giulia, et al. "The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists." Pharmacological Research 182 (2022):. 106320.
[21] Nevola, Riccardo, et al. "GLP-1 receptor agonists in non-alcoholic fatty liver disease: current evidence and future perspectives." International Journal of Molecular Sciences 24.2 (2023): 1703.
[22] Wharton, Sean, et al. "Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity." New England Journal of Medicine (2023).
[23] Jansen, Kirsten M., et al. "Impact of GLP-1 receptor agonist versus omega-3 fatty acids supplement on obesity-induced alterations of mitochondrial respiration." Frontiers in Endocrinology 14 (2023): 1098391.
[24] Mekhaimar, Menatalla, et al. "Glp-1 receptor agonists are associated with weight loss among lvad patients with diabetes and obesity." Journal of Cardiac Failure 29.4 (2023): 617.
[25] Cardoso, Luiz EM, et al. "Treatment with semaglutide, a GLP-1 receptor agonist, improves extracellular matrix remodeling in the pancreatic islet of diet-induced obese mice." Life Sciences 319 (2023): 121502.
[26] Palanca, Ana, et al. "Real-World Evaluation of GLP-1 Receptor Agonist Therapy Persistence, Adherence and Therapeutic Inertia Among Obese Adults with Type 2 Diabetes." Diabetes Therapy 14.4 (2023): 723-736.
[27] Luli, Alex, et al. "Barriers to Obtaining GLP-1 Receptor Agonists and SGLT2 Inhibitors in a Free Clinic Population With Type 2 Diabetes and Cardiovascular Disease." Journal of Contemporary Pharmacy Practice 70.1 (2023): 9-13.
[28] Mahtta, Dhruv, et al. "Utilization rates of SGLT2 inhibitors and GLP-1 receptor agonists and their facility-level variation among patients with atherosclerotic cardiovascular disease and type 2 diabetes: insights from the Department of Veterans Affairs." Diabetes Care 45.2 (2022): 372-380.
[29] Chen, Jian, et al. "Liraglutide activates autophagy via GLP-1R to improve functional recovery after spinal cord injury." Oncotarget 8.49 (2017): 85949.
[30] Han, Yi, Yingjie Li, and Bing He. "GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis." Reproductive biomedicine online 39.2 (2019): 332-342.
[31] Tao, Xin, et al. "Effects of metformin and Exenatide on insulin resistance and AMPKα-SIRT1 molecular pathway in PCOS rats." Journal of ovarian research 12.1 (2019): 1-8.
[32] Bednarz, Krzysztof, et al. "The role of glp-1 receptor agonists in insulin resistance with concomitant obesity treatment in polycystic ovary syndrome. " International Journal of Molecular Sciences 23.8 (2022): 4334.

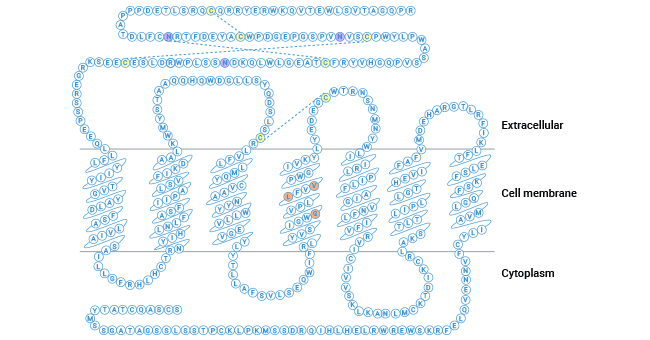
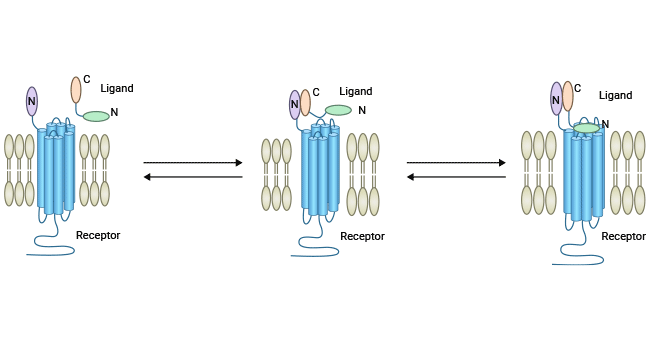
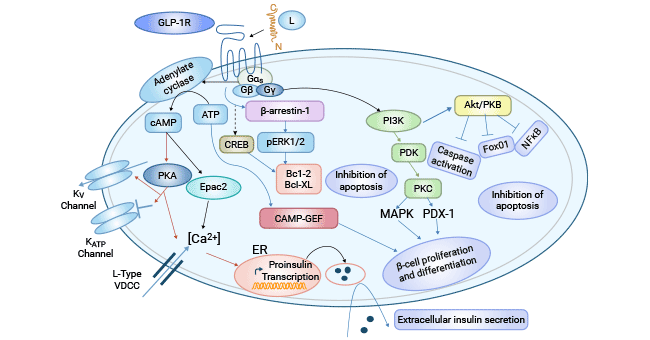
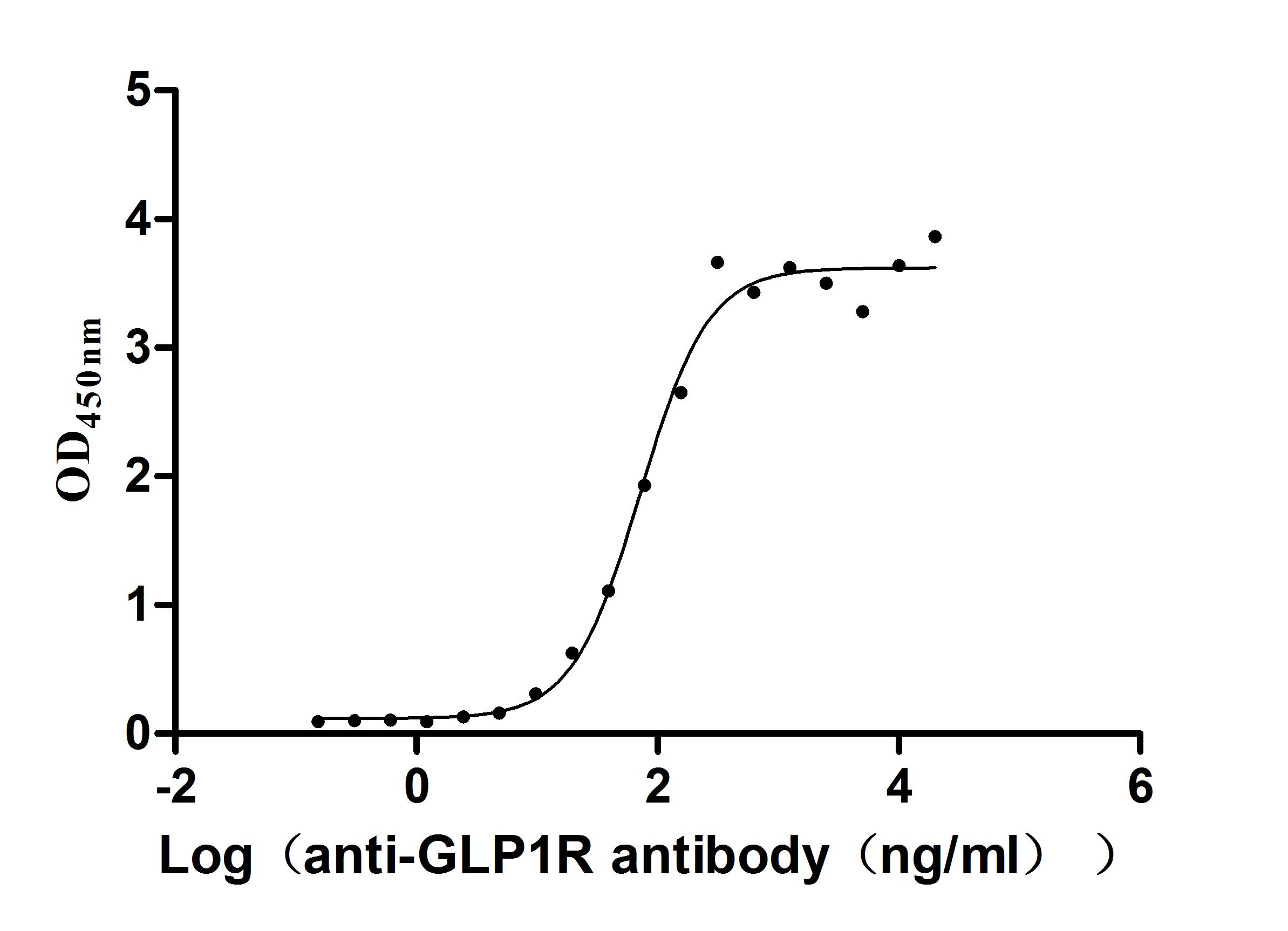
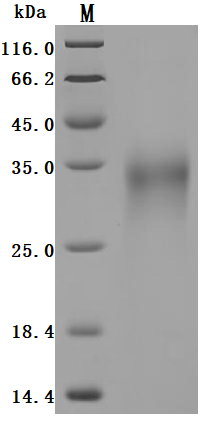
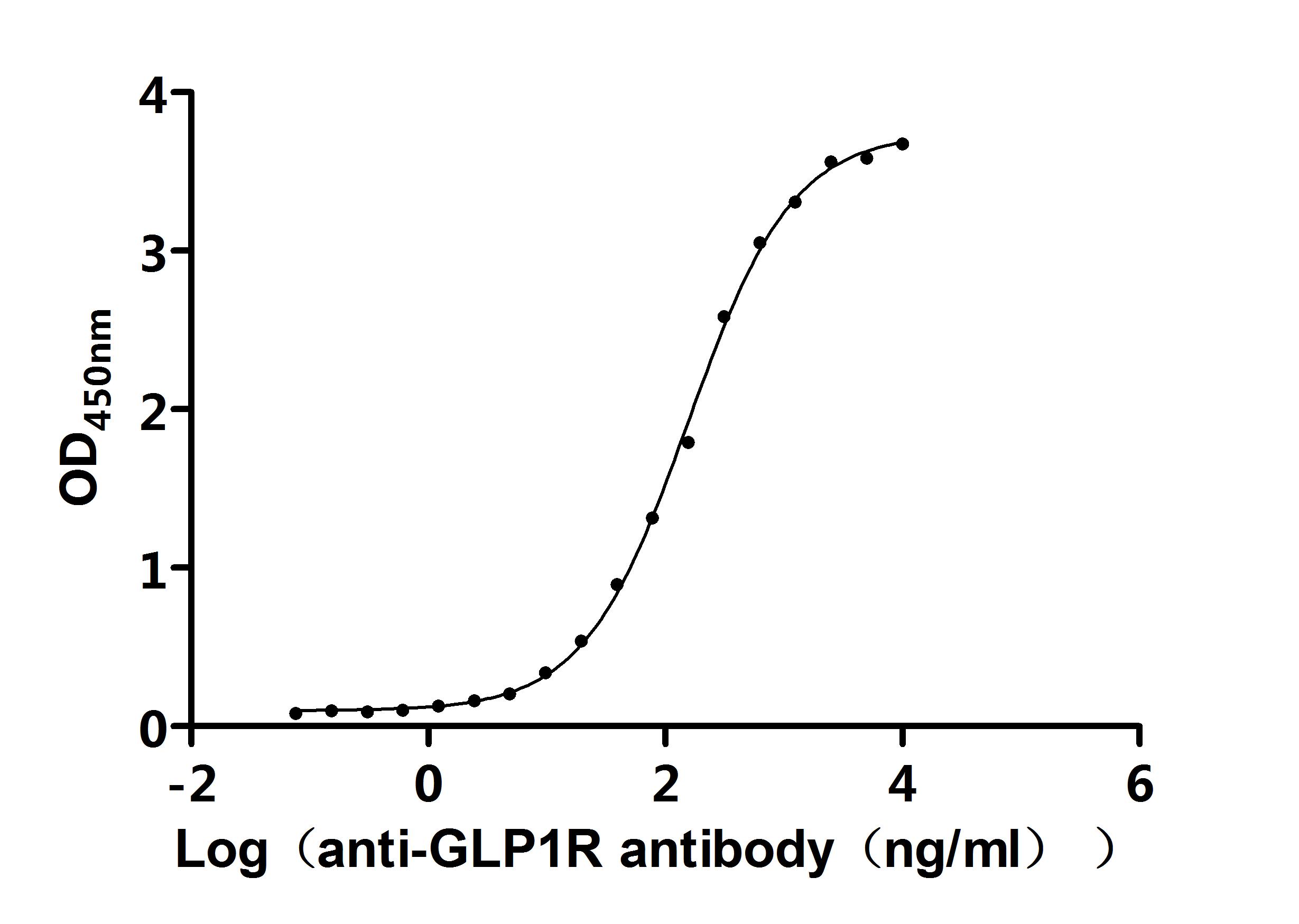
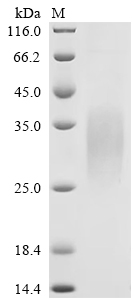

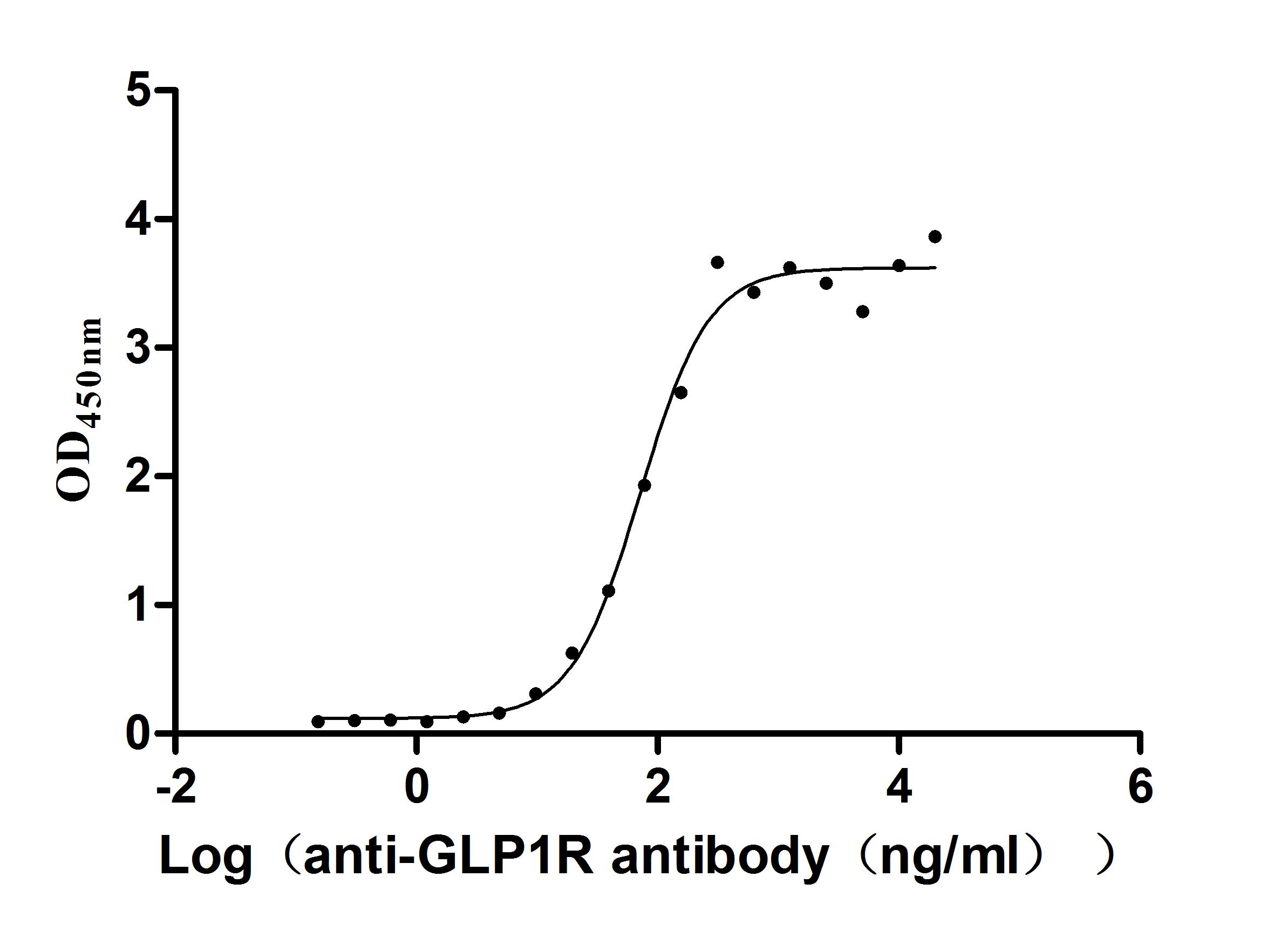
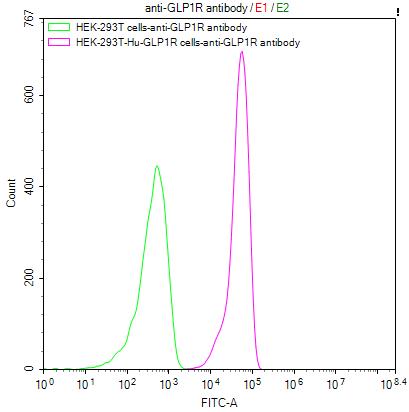
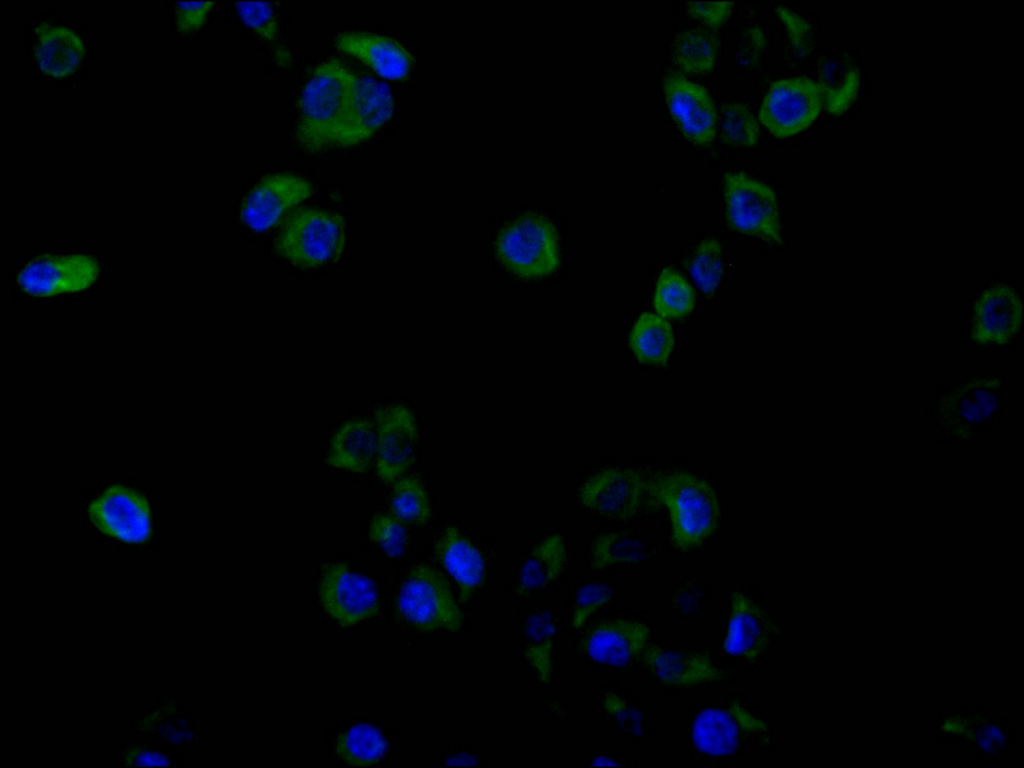
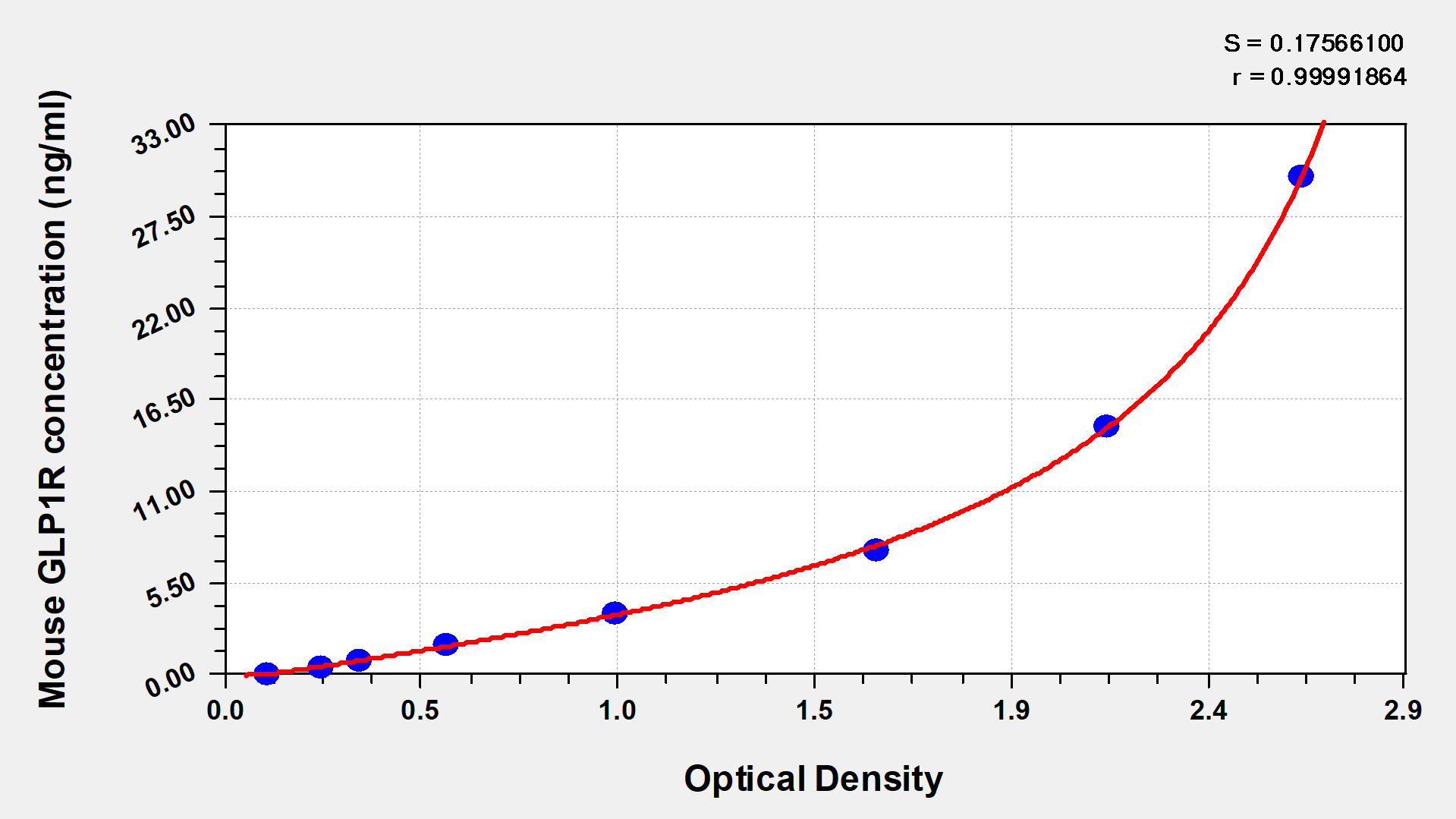



Comments
Leave a Comment