On March 15, 2024, the Journal of Affective Disorders published a study delving into the molecular mechanisms of post-traumatic stress disorder (PTSD) and major depression (MDD). The research revealed a notable decrease in the expression levels of DPEP3, GNAQ, and PACDIN2 in individuals with both conditions. Particularly, DPEP3, an enzyme specialized in breaking down dipeptides, has emerged as a key player in various fields. Recent studies have highlighted its significance, from addressing abnormal protein expression in male semen during COVID-19 recovery to tackling issues in male reproduction and cancer research. [1].
For example, in targeting DPEP3-expressing ovarian cancer cells, scientists have developed SC-003, a novel therapeutic agent. This drug has shown promise in inducing tumor regression by specifically targeting DPEP3-expressing cells, including those resistant to conventional treatments like HGSC PDX models. Moreover, its effectiveness can be further enhanced when used in combination with anti-PD 1 antibodies [2]. These findings emphasize the potential of DPEP3 as a promising target for therapeutic research, paving the way for the development of novel drugs to address challenges in male fertility, cancer, and other diseases.
1. What is DPEP3?
1.1 The Structure of DPEP3
Dipeptidase-3 (DPEP3) is part of the membrane-bound dipeptidase family (DPEP), alongside members such as Dipeptidase 1 (DPEP1) and Dipeptidase 2 (DPEP2). These proteins are part of the GPI-anchored ankyrin family, immobilized on cell membranes by glycosylphosphatidylinositol, and involved in dipeptide hydrolysis. Both DPEP1 and DPEP3 share a similar extracellular structure known as TIM (β/α)8-barrel, featuring eight helices surrounding eight β-folds, suggesting catalytic capability [2-5].
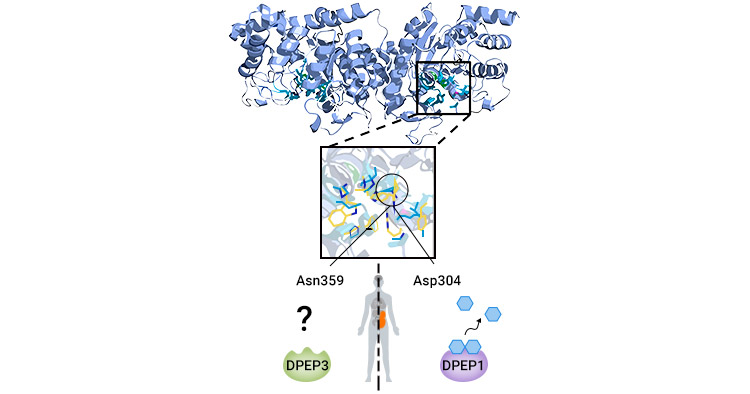
In recent research, scientists have unveiled the atomic structure of DPEP3 and its interaction with the SC-003 Fab fragment. Notably, DPEP3 differs from DPEP1 and DPEP2 at key active site positions: substitution of histidine with tyrosine at position 269 reduces β zinc binding, potentially impeding substrate binding, while substitution of aspartic acid with asparagine at position 359 prevents water/hydroxyl group activation, leading to impaired dipeptide and substrate degradation in vitro. Consequently, unlike DPEP1 and DPEP2, DPEP3 may necessitate cofactors for activity or remain inactive (Figure 1) [2-5].
Figure 1. Structure of DPEP3 [3]
1.2 The Expression and Function of DPEP3
The DPEP3 protein exhibits high conservation across species, including humans, mice, and other mammals, with its mRNA exclusively expressed in testicular tissue. Research suggests that DPEP3 may play a vital role in testicular function and the normal development of the fertility system. Despite its predominant expression in the testes, aberrant expression of DPEP3 has been observed in certain tumors [2-5]
Moreover, the DPEP family primarily functions in the hydrolysis of dipeptides. For instance, DPEP1 and DPEP2 have been implicated in converting leukotriene D4 (LTD4) to leukotriene E4 (LTE4), thereby modulating leukotriene activity, while DPEP1 and DPEP3 possess the ability to cleave cysteinyl-glycine to generate cysteine and glycine. However, comprehensive understanding of the DPEP family, including its structure and broader biological functions, remains limited and requires further investigation [2-5].
2. How's the DPEP3 Related Mechanisms of Action?
The DPEP family stands as a significant subset within the GPI family. Scholarly research confirms the exclusive expression of DPEP3 in the testes, sparking investigations into its potential regulatory role in sperm reproduction and fertility. Furthermore, researchers are delving into the plausible involvement of DPEP3 expression in inflammatory responses.
2.1 DPEP3-Related Fertility Regulatory Mechanisms
The DPEP family, an integral part of the GPI family, plays a crucial role in fertility processes. Studies have demonstrated that DPEP3 interacts with the testis-specific protein phosphatidylinositol ankyrin TEX101, impacting male fertility. For instance, research on spermatogenic mechanisms reveals that DPEP3 forms a complex with TEX101, essential for sperm mobility and male fertility in mice. TEX101, expressed during gametogenesis, is vital for sperm production. These findings underscore DPEP3's significance in male reproduction and shed light on spermatogenic mechanisms. Additionally, studies indicate independent regulation of DPEP family members' expression in the testes, as evidenced by unaffected levels of DPEP3 and Adam3 in the absence of TEX101 [6–8]
2.2 DPEP3-Related Inflammatory Regulatory Mechanisms
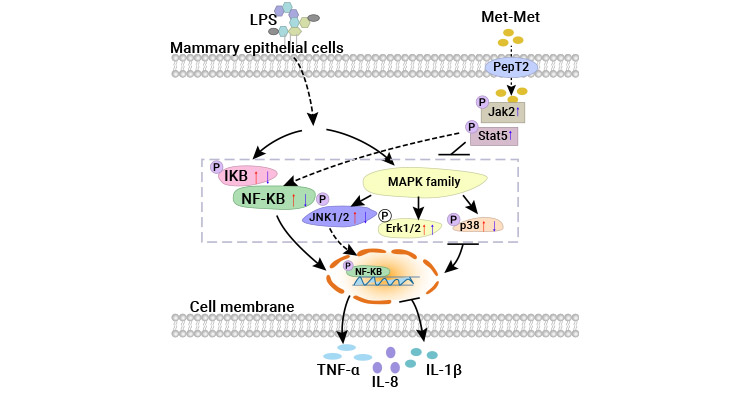
Moreover, the DPEP enzyme family, comprising key enzymes, is implicated in inflammation. Studies indicate that methionine dipeptide (Met-Met) can be transported to the breast via peptide transporter 2 (PepT2), where aminopeptidase N (APN) partially breaks it down into free Met-Met, potentially exerting anti-inflammatory effects. While enzymes like γ-glutamyltransferase (γ-GT), dipeptidase 1 (DPEP1), dipeptidase 2 (DPEP2), and DPEP3 are mentioned, their direct role in Met-Met action remains unclear. Nevertheless, these enzymes are crucial in cellular metabolism and protein degradation, likely influencing Met-Met's metabolic pathway and effects (Figure 2) [9]. Additionally, studies on the biosynthetic pathway of maverin conjugate MCTR have identified key enzymes, including DPEP, pivotal in converting MCTR1 to MCTR3 in human macrophages. These insights offer potential for innovative therapeutic strategies in inflammatory diseases [10].
Figure 2. Dipeptidase might be involved in the metabolic pathway of Met-Met [10]
3. DPEP3 and Disease Related Research
"Dipeptidase" enzymes are specialized in enzymatically cleaving dipeptides. While the precise biological role of DPEP3 is still being elucidated, research suggests its potential implications in reproduction. Moreover, recent findings indicate DPEP3's association with tumor development and progression. Consequently, targeting DPEP3 presents a promising avenue for therapeutic research across various diseases.
3.1 DPEP3 and Fertility Research
In a study on semen proteins of men recovering from COVID-19, several key proteins linked to male fertility function were found dysregulated. Among them are SEMG1, ODF2, CD59, PSAP, DPEP3, SPA17, NRP1, RAB6B, RAB3A, MENT, and IBP5. Neuropilin-1 (NRP1) plays a vital role in spermatogenic stem cell differentiation, and reduced levels may affect fertility. DPEP3 downregulation could disrupt its interaction with TEX101, impacting testicular function. Prosaposin (PSAP) at the low level may indicate COVID-19-induced harm to male fertility organs, suggesting disruptions in crucial regulatory factors [11].
3.2 DPEP3 and Tumor Research
3.2.1 DPEP3 and Ovarian Cancer Research
In a study involving the antibody drug conjugate (SC-003) targeting DPEP3, significant anti-tumor effects were observed in patient-derived xenotransplantation models of high-grade serous ovarian cancer (HGSC). Patient-derived xenograft (PDX) models were crucial for identifying treatment targets and assessing drug efficacy. Through analysis of tumor-initiating cells (TICs) in the HGSC PDX model, researchers found that DPEP3 was enriched in TICs of both platinum-sensitive and drug-resistant HGSC, localized on the cell membrane, and detectable via flow cytometry and immunohistochemistry. Conversely, DPEP3 expression was minimal or absent in most normal adult tissues, suggesting its potential as a promising antibody drug target. [2,12–13].
3.2.2 DPEP3 and Non-Small Cell Lung Cancer Research
In a study, researchers examined the expression of carcinoembryonic antigen (CTA) proteins in non-small cell lung cancer (NSCLC) and their relationship with immune cells. Eight CTAs (DPEP3, EZHIP, MAGEA4, MAGEB2, MAGEC2, PAGE1, PRAME, and TKTL1) were analyzed in NSCLC tissues. Results showed that most NSCLC patients had one or more CTAs expressed, which correlated with immune cell distribution. While the clinical significance remains unclear, these CTAs might affect immune responses in tumor tissue. This study contributes to our understanding of immune responses in NSCLC and suggests CTAs as potential targets for NSCLC immunotherapy [14].
3.3 DPEP3 and Other Related Research
DPEP3 is implicated in various fertility studies, including investigations into varicoceles, a common cause of male infertility with uncertain effects on semen quality. Research suggests that varicoceles can impact semen metabolism, but microsurgery may reverse these effects. Interestingly, levels of DPEP3 increase after microsurgery, offering a new avenue for early diagnosis and treatment of varicoceles [15].
Furthermore, DPEP3 is linked to rheumatoid arthritis (RA), a condition that significantly affects patients' quality of life. Studies have identified lower expression levels of certain genes, including DPEP3, in colon cells of RA patients. This abnormal gene expression in the intestine may contribute to disease development and symptom severity. These findings highlight the potential involvement of DPEP3 in both fertility health and RA research [16].
4. The Prospect of DPEP3 in Clinical Drug Research
Research on DPEP3 is still in its early stages, and clinical research targets are not yet in development. Nevertheless, existing literature suggests its potential for clinical applications. Firstly, in fertility aspect, DPEP3 research could address male infertility issues by uncovering its role in fertility disorders, paving the way for innovative treatments. Secondly, drugs targeting DPEP3 may hold promise in cancer treatment, particularly for ovarian cancer and non-small cell lung cancer. Additionally, DPEP3 is linked to autoimmune research like rheumatoid arthritis, offering insights into novel therapeutic strategies. Overall, DPEP3 emerges as a promising therapeutic target with implications for various diseases, shaping future clinical applications and research strategies.
In Conclusion:
Dipeptidase 3 (DPEP3) is an enzyme involved in the hydrolysis of dipeptides. Its diverse roles include implications in male fertility health, tumor biology, and autoimmune diseases like rheumatoid arthritis. Studies suggest its potential as a therapeutic target for addressing male infertility and treating cancers such as ovarian and lung cancers. While still in early stages, research on DPEP3 holds promise for future clinical applications.
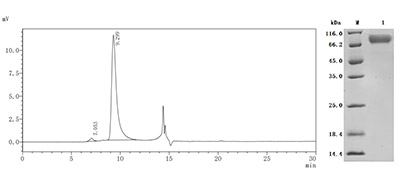
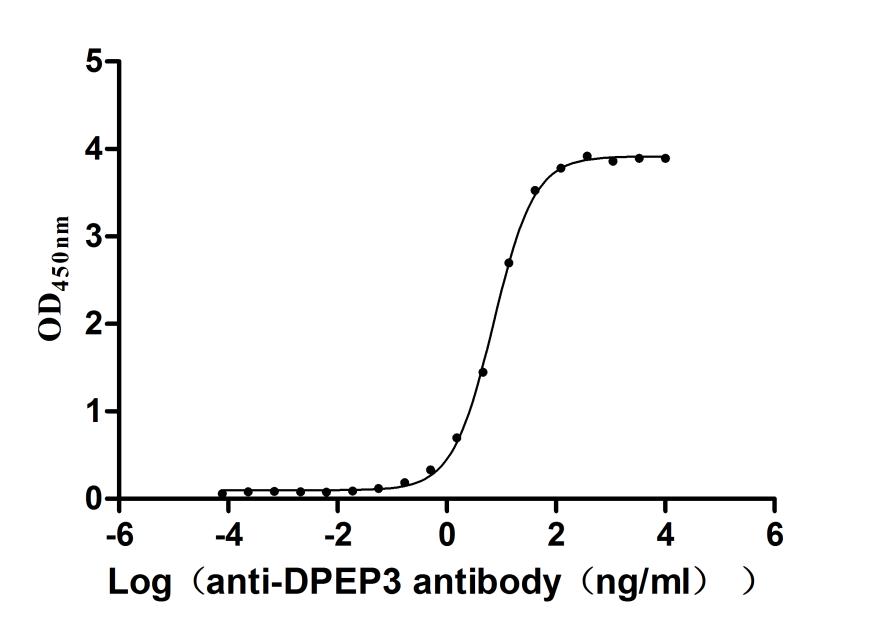
5. CUSABIO DPEP3 Recombinant Proteins for Research Use
To fully support pharmaceutical companies in their clinical research on DPEP3 such as male fertility and cancer, CUSABIO launched DPEP3 active protein products to aid your investigation of the mechanisms of DPEP3 or its potential clinical value.
References
[1] Zhang, Haofuzi, Peng Luo, and Xiaofan Jiang. "Comprehensive bioinformatics analysis of co-expressed genes of post-traumatic stress disorder and major depressive disorder." Journal of Affective Disorders 349 (2024): 541-551.
[2] Wiedemeyer, Wolf R., et al. "Abstract NT-113: SC-003, AN ANTIBODY-DRUG CONJUGATE TARGETING DIPEPTIDASE 3, EXHIBITS POTENT ANTI-TUMOR ACTIVITY IN PATIENT-DERIVED XENOGRAFT MODELS OF HIGH GRADE SEROUS OVARIAN CANCER." Clinical Cancer Research 25.22_Supplement (2019): NT-113.
[3] Hayashi, Kristyn, et al. "Structure of human DPEP3 in complex with the SC-003 antibody Fab fragment reveals basis for lack of dipeptidase activity." Journal of Structural Biology 211.1 (2020): 107512.
[4] Choudhury, Saurav Roy, et al. "Dipeptidase-1 is an adhesion receptor for neutrophil recruitment in lungs and liver." Cell 178.5 (2019): 1205-1221.
[5] Wang, Yuanyi, et al. "Dipeptidase‑2 is a prognostic marker in lung adenocarcinoma that is correlated with its sensitivity to cisplatin." Oncology Reports 50.2 (2023): 1-15.
[6] Schiza, Christina, et al. "Discovery of a human testis-specific protein complex TEX101-DPEP3 and selection of its disrupting antibodies." Molecular & Cellular Proteomics 17.12 (2018): 2480-2495.
[7] Yoshitake, Hiroshi, et al. "Molecular characterization and expression of dipeptidase 3, a testis-specific membrane-bound dipeptidase: complex formation with TEX101, a germ-cell-specific antigen in the mouse testis." Journal of reproductive immunology 90.2 (2011): 202-213.
[8] Endo, Shuichiro, et al. "TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells." Scientific reports 6.1 (2016): 23616.
[9] Lan, Wei, et al. "Methionyl-methionine exerts anti-inflammatory effects through the JAK2-STAT5-NF-κB and MAPK signaling pathways in bovine mammary epithelial ce lls." Journal of agricultural and food chemistry 68.47 (2020): 13742-13750.
[10] Tang, Shi, et al. "Maresins: specialized proresolving lipid mediators and their potential role in inflammatory-related diseases." Mediators of inflammation 2018 (2018).
[11] Ghosh, Susmita, et al. "Semen proteomics of COVID-19 convalescent men reveals disruption of key biological pathways relevant to male reproductive function." ACS omega 7.10 (2022): 8601-8612.
[12] Wiedemeyer, Wolf R., et al. "Abstract NT-113: SC-003, AN ANTIBODY-DRUG CONJUGATE TARGETING DIPEPTIDASE 3, EXHIBITS POTENT ANTI-TUMOR ACTIVITY IN PATIENT-DERIVED XENOGRAFT MODELS OF HIGH GRADE SEROUS OVARIAN CANCER." Clinical Cancer Research 25.22_Supplement (2019): NT-113.
[13] Fu, Ruisi, et al. "Development of an antibody-drug conjugate panel targeting high grade serous ovarian cancer." Cancer Research 83.7_Supplement (2023): 455-455.
[14] Hikmet, Feria, et al. "Expression of cancer–testis antigens in the immune microenvironment of non‐small cell lung cancer." Molecular Oncology 17.12 (2023): 2603-2617.
[15] Zhang, Xinzong, et al. "Effects of varicocele and microsurgical varicocelectomy on the metabolites in semen." Scientific Reports 12.1 (2022): 5179.
[16] Lai, Wenjia, et al. "Lycium barbarum polysaccharide modulates gut microbiota to alleviate rheumatoid arthritis in a rat model." npj Science of Food 6.1 (2022): 34.
CUSABIO team. Dipeptidase 3 (DPEP3): an Emerging Member of the DPEP Family, a Potential Therapeutic Target in Male Fertility or Tumor Research!. https://www.cusabio.com/c-21168.html




Comments
Leave a Comment