Explore the potential of our Recombinant Human CCL8 protein, an essential research tool for immunology-focused investigations. This C-C motif chemokine 8, also known as CCL8, is produced in E. coli and encompasses the 24-99aa expression region of the full-length mature protein. The tag-free protein is supplied in lyophilized powder form, allowing for straightforward reconstitution with sterile water or buffer for a wide range of experimental applications.
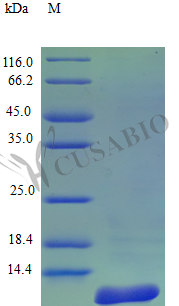
Committed to quality and performance, our Recombinant Human CCL8 protein exhibits a purity of >96% as determined by SDS-PAGE and HPLC analysis. Furthermore, endotoxin levels are maintained below 1.0 EU/µg, as determined by the LAL method. The protein demonstrates full biological activity, as determined by a chemotaxis bioassay using human peripheral blood monocytes, with an effective concentration range of 10-100 ng/mL.
Significant research has been conducted to understand the function and relevance of CCL8 (C-C motif chemokine 8) in various biological processes and diseases. CCL8, a member of the CC chemokine family, was first characterized by Proost et al. (1996)[1], who elucidated its role as a chemoattractant for monocytes, eosinophils, and basophils. The involvement of CCL8 in the recruitment of leukocytes during inflammation was further supported by Van Coillie et al. (1999)[2], highlighting its role in immune responses. Menten et al. (2002)[3] explored the relationship between CCL8 and HIV-1 infection, demonstrating that CCL8 serves as a potent inhibitor of the R5 strains of HIV-1, thereby suggesting its potential role in controlling HIV-1 infection. CCL8's association with cancer was demonstrated by Negus et al. (1995)[4], who identified the overexpression of CCL8 in human melanoma cell lines, and it has since been implicated in various cancer types. Furthermore, a study by Bandapalli et al. (2014)[5] revealed the involvement of CCL8 in colorectal cancer progression and its potential as a diagnostic and therapeutic target.
References:
1. Proost P, et al. Human and bovine granulocytes express a natural IL-8 inhibitor: characterization of the cDNA coding for the human homolog. Eur J Immunol. 1996;26(10): 2388-93.
2. Van Coillie E, et al. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10(1): 61-86.
3. Menten P, et al. The LD78beta isoform of MIP-1alpha is the most potent CCR5 agonist and HIV-1-inhibiting chemokine. J Clin Invest. 2002;110(4): 587-94.
4. Negus RP, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95(5): 2391-6.
5. Bandapalli OR, et al. Transcriptional activation of CCL8 by TGF-β1-SMAD/SMAD4 and IFN-γ-NF-κB in colorectal cancer stroma. Int J Cancer. 2014;134(3): 517-28.