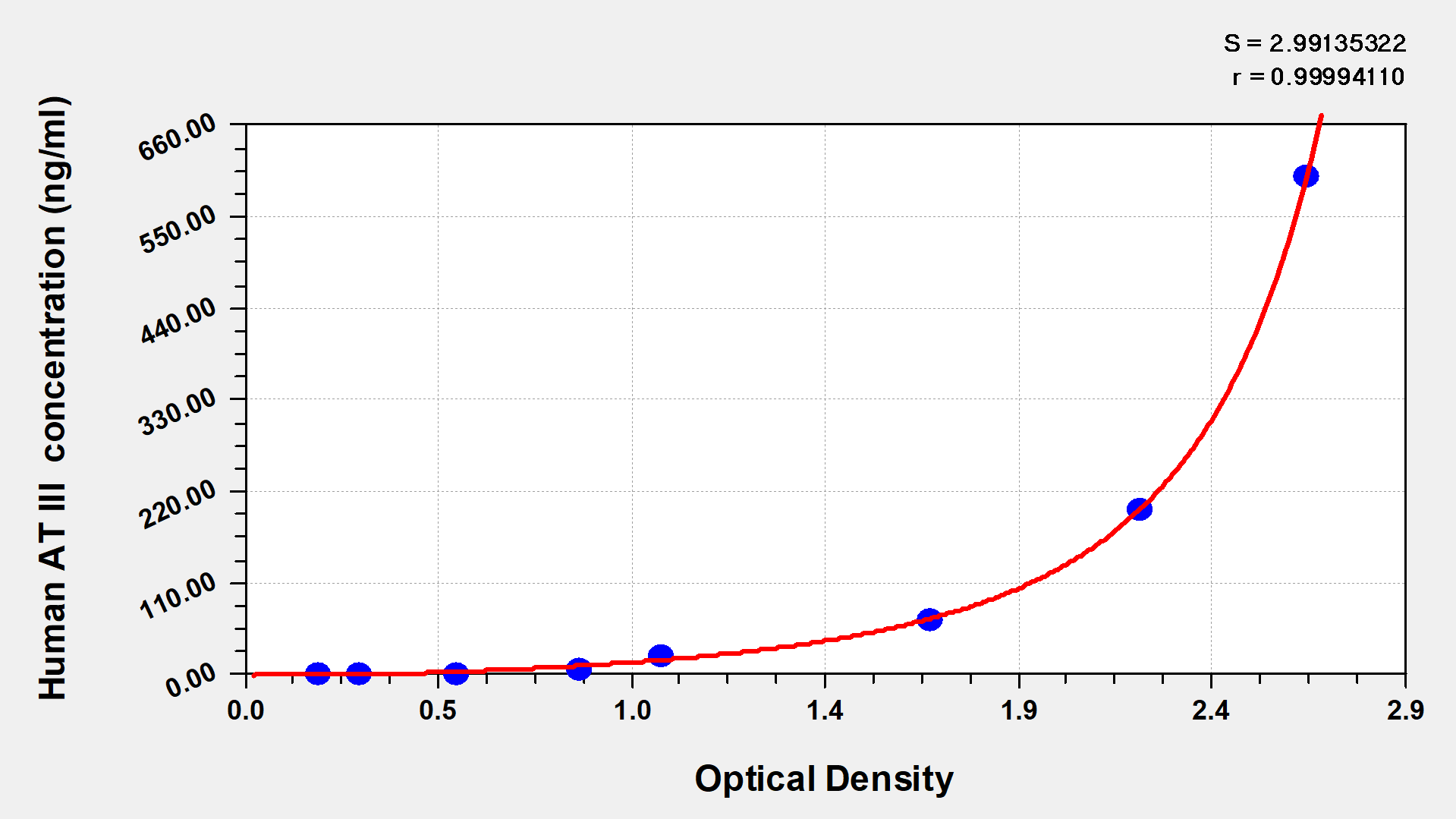
The human Antithrombin III (AT III) ELISA kit is suitable for the quantitative determination of human AT III in different sample types, including serum, plasma, and tissue homogenates. This assay employs the bi-antibody sandwich technique and enzyme-substrate chromogenic reaction to quantify antigen levels in the sample. The amount of synthesized colored products is positively related to the analyte of interest in the sample.
AT III, encoded by the gene SERPINC1, is a small glycoprotein anticoagulant that inactivates several enzymes of the coagulation system like thrombin thus blocking the formation of aberrant blood clots. It plays an important role in the maintenance of a healthy balance between bleeding and clotting. Hereditary or acquired AT III deficiency leads to thromboembolism, which can block blood flow and damage organs. In addition to the anticoagulant effect, AT III also has an anti-inflammatory role. AT III inhibits inflammation through a coagulant-dependent or -independent effect.