HCP Detection ELISA Kit Now Available!
- High Sensitivity
- High Stability
- Broad Antibody Coverage
What are Host Cell Proteins (HCP)?
Host Cell Proteins (HCP) are residual protein impurities derived from host cells (such as yeast, E. coli, mammalian cells, insect cells, etc.) during the production of biopharmaceuticals. These impurities may accompany the target drug protein during cell culture, fermentation, or expression. Despite purification processes, they may still remain in the final product, potentially affecting the safety and efficacy of the drug.
The Importance of HCP Detection
The potential risks posed by HCP residues include:
- Triggering immune responses in patients (e.g., fever, allergies)
- Compromising drug stability and efficacy
- Leading to regulatory approval failures (FDA, EMA, CFDA, and ICH all specify HCP threshold requirements)
Accurate measurement of HCP residue levels is not only critical for ensuring clinical drug safety but also serves as a cornerstone for optimizing production processes, reducing R&D risks, and ensuring compliance with global regulatory standards.
Common Methods for HCP Detection
| Method |
Technical Features |
Applications |
| ELISA (Enzyme-Linked Immunosorbent Assay) |
- Based on a double-antibody sandwich method, high sensitivity (detection and quantification limits as low as ng/mL);
- Simple operation, standardized kits available;
- Relies on high-quality antibodies and specificity, requiring antibody coverage validation.
|
- Quality control, process optimization, final product release testing;
- Recommended by regulatory guidelines (e.g., USP, ICH).
|
| Mass Spectrometry (LC-MS/MS) |
- High resolution, capable of identifying specific HCP types, antibody-independent, comprehensive coverage;
- Requires specialized equipment and data processing support.
|
- Process development for HCP characterization, high-risk HCP analysis (e.g., PLBL2), orthogonal validation of ELISA results.
|
| Western Blot |
- Visualized results, detects specific HCPs, provides molecular weight information;
- Lower sensitivity (requires ng-level protein);
- Limited to known HCPs.
|
- Screening specific HCPs, validating ELISA results, monitoring HCP in process intermediates.
|
| Two-Dimensional Gel Electrophoresis (2D-PAGE) |
- Combines isoelectric focusing and SDS-PAGE to separate HCPs and generate 2D maps;
- Cannot quantify directly, requires mass spectrometry for identification.
|
- HCP characterization during process development, preliminary analysis of complex samples.
|
| Capillary Electrophoresis (CE) |
- Fast, requires minimal sample volume (nL level), highly automated;
- Lower sensitivity (requires μg-level protein), often used in combination with other methods.
|
- Rapid HCP screening, small-scale sample analysis, quantification with mass spectrometry.
|
| HPLC |
- Useful for evaluating HCP removal efficiency, standardized operation;
- Cannot distinguish HCP types, depends on elution conditions.
|
- Monitoring purity of process intermediates, optimizing HCP removal processes.
|
CUSABIO's HCP Detection ELISA Kit
As the industry "gold standard," ELISA is widely recognized by global regulatory agencies for its simplicity, high sensitivity, and reliable results. Leveraging a mature enzyme-linked immunosorbent assay platform, CUSABIO has developed a series of HCP detection kits suitable for various host cells. These kits enable precise quantification of HCP residues in production stages (cell culture supernatants, purification intermediates, final products) for expression systems such as CHO, HEK293, and E. coli, providing reliable analytical tools for biopharmaceutical process development and quality control.
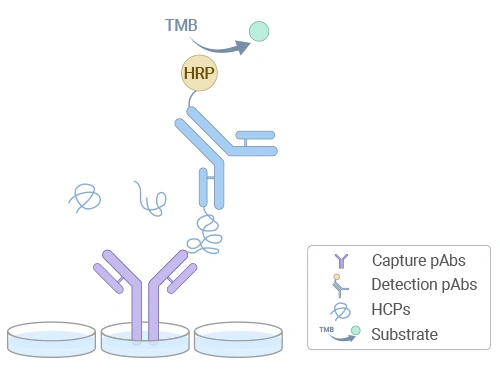
Schematic diagram of HCP residue detection by ELISA
Product Advantages
High Sensitivity
Uses high-titer antibodies (titer ≥106) combined with affinity purification, achieving detection limits as low as ng/mL.
Broad Antibody Coverage
Employs high-affinity polyclonal antibodies to recognize most host cell proteins, ensuring comprehensive detection.
High Stability
Incorporates broad-spectrum protein stabilizers and microplate processing technology, minimizing intra- and inter-batch variability.
Strong Compatibility
Optimized buffer system reduces interference from complex matrices.
End-to-End Solution
Covers detection across all production stages, compliant with global regulatory standards.
Product List
For different types of test samples, different detection kits are set up. Please click on the product details page to select the appropriate kit based on your experimental needs.
| Code |
Product Name |
| CSB-EQ33265PY |
Pichia Yeast host cell protein (HCP) Residue ELISA Kit |
| CSB-EQ33264HEK |
293 Host Cell Protein (HCP) Residue ELISA Kit |
| CSB-EQ33263CHO |
Chinese Hamster Ovary (CHO) Host Cell Protein (HCP) Residue ELISA Kit |
| CSB-EQ33262CHO |
Chinese Hamster Ovary (CHO) Host Cell Protein (HCP) Residue ELISA Kit |
| CSB-EQ33261ECO |
E. coli Host Cell Protein (HCP) Residue ELISA Kit |
FAQ
Q1: What sample types are compatible with this kit?
A: This kit can quantitatively detect HCP residues in culture supernatants, protein purification intermediates, and final products. It is suitable for biopharmaceutical R&D, process optimization, and quality control.
Q2: What detection principle does this kit use?
A: The kit employs a double-antibody sandwich method, forming an "antibody-antigen-antibody" complex with coated antibodies and HRP-labeled detection antibodies. HCP residue levels are quantified via TMB color development and OD measurement.
Q3: What are the storage conditions for kit components? Can opened reagents be reused?
A: All reagents (excluding the sealing film) must be stored sealed at 2–8 °C. Unused microplate strips may be resealed with the sealing film and returned to the foil bag for storage at 4 °C. All reagents and components should be used within one week after opening.
Q4: Why is spike recovery testing necessary? How is it performed?
A: The spike recovery experiment is used to evaluate whether the sample matrix interferes with the detection results. For example, for product CSB-EQ33263CHO, it is recommended to mix diluted standard S1 (400 ng/mL) with the test sample at a ratio of 1:3 (such as adding 25 μL of standard S1 with or without a concentration of 400 ng/mL into 75 μL of test solution). The recovery rate should then be calculated. An ideal recovery rate should fall within 80–120% to ensure data reliability.






