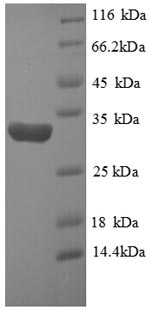
Recombinant Human HIF1A protein is a prokaryotic system E.coli expressed Partial protein. To make this HIF1A recombinant protein, the HIF1A gene is synthesized and cloned into the E.coli system expression vector, and then it was transformed into protein-expressing host E Coli for expression, the other steps are testing for the identification of recombinant protein, large scale production and target protein Isolation and purification. The purity of the final HIF1A protein is 90%+ as determined by SDS-PAGE.
HIF1A (also known as BHLHE78 or MOP1) is a protein encoding gene that provides an instruction in making a protein named hypoxia-inducible factor 1-alpha (Short name is HIF1-alpha) or ARNT-interacting protein. The protein encoded by this gene is the alpha subunit of transcription factor hypoxia-inducible factor-1 (HIF-1), which is a heterodimer composed of an alpha and a beta subunit. HIF-1β subunit is constitutively expressed. Oxygen-dependent HIF-α subunit has three α isoforms, including HIF-1alpha, HIF-2alpha or HIF-3alpha. Among of them, HIF-1α is the one playing a major role in hypoxia signaling.