Discover the potential of Recombinant Human Parkinson Disease Protein 7 (PARK7) in cancer research with our premium quality product. PARK7, also known as DJ-1, has been implicated in various cellular processes, including oxidative stress response, cell survival, and transcriptional regulation. Its multifaceted role in cancer progression and response to therapies makes it an intriguing target for researchers in the field.
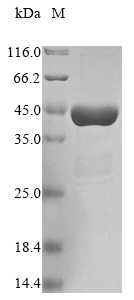
Our Recombinant Human PARK7 protein is expressed in an E.coli system, ensuring a reliable and high-quality source of protein for your research needs. The partial protein sequence (1-188aa) is delivered in a tag-free format, providing an unmodified protein that closely mimics its native form. Boasting a purity greater than 90% as determined by SDS-PAGE, our PARK7 protein ensures consistent and reproducible results across various applications. Available in both liquid and lyophilized powder forms, our Recombinant Human PARK7 protein is tailored to meet the diverse requirements of your cancer research endeavors.