ZG16B has recently emerged as a novel oncogene based on comprehensive bioinformatics analysis. Apparently, treatment of malignancies has experienced immense progress through immunotherapy. But the success of immunotherapy has not translated to the treatment of pancreatic cancer since pancreatic cancer is usually non-immunogenic. Besides, early invasive metastasis and drug resistance of pancreatic cancer are the main reasons affecting its clinical outcome. Therefore, the identification of new targets and therapeutic approaches would be useful in the battle against this deadly disease. Intriguingly, numerous studies have revealed that ZG16B is aberrantly expressed in multiple cancers, especially in pancreatic cancer. It suggests that ZG16B might serve as a promising biomarker for tumor diagnosis, or even a therapeutic target. Moreover, it is worth noting that several ZG16B antibody drugs are in the clinical stage, mainly for pancreatic and ovarian cancer treatment. Overall, ZG16B as new tumor biomarker will provide a new idea for the diagnosis and treatment of pancreatic cancer.
1. What ZG16B?
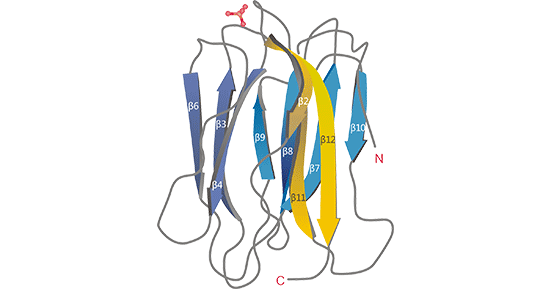
Zymogen granule protein 16 homolog B (ZG16B) is a newly discovered oncogene that belongs to a paralog of ZG16A [1]. It has been noted that ZG16B is a mammalian lectin containing a β-prism fold structure, which contributes to the ability to regulate cell adhesion, metastasis, apoptosis, angiogenesis, and cell-cell interactions to recognize pathogens (Figure 1) [2,23]. ZG16B is located at the human chromosome 16p13.3 locus, a 721 bp cDNA sequence, and has a 196 amino acid. The open reading frame region of ZG16B contains a nucleic acid structure similar to that of human salivary proteins [3]. ZG16B was first found to be highly expressed in pancreatic cancer and played an important oncogenic role, which led to its identification as a pancreatic adenocarcinoma upregulated factor (PAUF). In pancreatic adenocarcinoma, ZG16B is activated by CXCR4, TPL2, β-catenin, TPL2/MEK/ERK, FAK/Scr, and other signaling pathways, which promotes pancreatic cancer angiogenesis [1-2].
Recent studies have confirmed that ZG16B is not just highly expressed in pancreatic cancer cells, but also in breast cancer, gastric cancer, and ovarian cancers. These findings demonstrate that it plays key roles in the proliferation, adhesion, migration, and invasion in different tumor cells [1-4]. ZG16B, as a new activator of human endothelial cells, has similar effects to vascular endothelial growth factor (VEGF), can promote angiogenesis and increase vascular permeability in vitro and in vivo [3]. However, ZG16B, a specific factor for pancreatic cancer, was found to promote the activation and maturation of Dendritic cells (DCs) through TLR4 signaling pathway to mediate the activation of the immune system; at the same time, it can also activate myeloid-derived suppressor cells (MDSCs) and promote tumor development, which has a dual role in immunotherapy [5-7].
Figure 1. ZG16B crystal structure [23]
2. How's the Regulation Mechanisms of ZG16B?
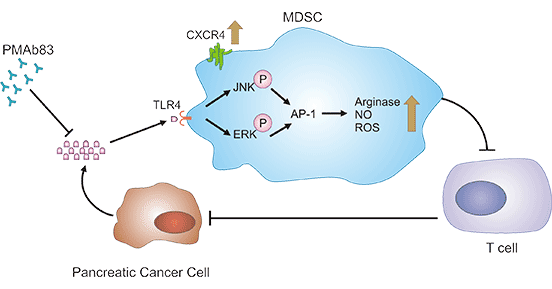
1) ZG16B activates the CXCR4 signaling pathway. When ZG16B is overexpressed, it induces the activation of regulatory kinases (ERK), the JNK, protein kinase signaling (AKT) and other pathways, ultimately actives the CXCR4 signaling pathway. In contrast, CXCR4 signaling pathway is inhibited as ZG16B is downregulated (Figure 2) [8].
2) ZG16B activates the β-catenin signaling pathway. ZG16B expression induces phosphorylation of ser-33/37/Thr-41 and ser-675 of β-catenin to activate the Akt/GSK-3β signaling pathway, and then β-catenin is translocated to the nucleus where it forms a complex with the T-cell factor/lymph node enhancer factor (TCF/LEF) family. This complex can activate the expression of β-catenin-responsive genes to accelerate the proliferation of pancreatic cancer cells [9].
3) ZG16B activates the TLR2 mediated TPL2/MEK/ERK signaling pathway. ZG16B is a ligand for TLR2 and TLR4 ligand that activates the TLR2-mediated TPL2/MEK/ERK signaling pathway. Meanwhile, the process will cause an increase in nuclear transcription factor AP-1, leading to the production of pro-oncogenic cytokines; when ZG16B-CXCR4-TLR2 downstream sensor protein kinase A (PKA) is activated, it can inhibit TLR2-induced activation of NF-κB, thereby curbing the activation of immune cells. Thus, ZG16B could promote the growth of pancreatic cancer cells as well as evade the surveillance of the immune system [10].
4) ZG16B activates the FAK signaling. ZG16B can activate the FAK-Src pathway to control the adhesion of pancreatic cancer cells. Both specific ZG16B antibodies and FAK inhibitors are effective in blocking the ZG16B-mediated adhesion. ZG16B can also regulate the FAK pathway to inhibit the apoptosis of pancreatic cancer cells [11].
Figure 2. ZG16B-related signaling pathways [8]
3. What's the Roles of ZG16B in Tumor Therapy?
3.1 ZG16B and Pancreatic Cancer
Pancreatic cancer remains one of the leading causes of cancer related death worldwide with an overall five-year survival of less than 5%. Adjuvant chemotherapy plays an important role in the comprehensive treatment of pancreatic cancer. However, the overall effectiveness of chemotherapy on pancreatic cancer is largely unsatisfactory. Gemcitabine is the only chemotherapy drug approved by the FDA for the treatment of pancreatic cancer. But less than 25% of pancreatic cancer patients are currently sensitive to Gemcitabine chemotherapy. Some data indicated that gemcitabine improves patient survival time by only 2 weeks.
Recent studies have shown that the proliferation, metastasis, and progression of transplanted tumors can be significantly reduced by injecting ZG16B inhibitors or knocking out the ZG16B gene in pancreatic cancer cells [12]. In fact, the first pancreatic cancer upregulatory factor monoclonal antibody, Ulenistamab (PBP1510) is in clinical phase 2 for pancreatic cancer treatment. Thus, targeting ZG16B is expected to provide a new option to patients with ZG16B/PAUF-positive pancreatic cancer.
3.2 ZG16B and Ovarian Cancer
Ovarian cancer is the deadliest reproductive malignancy because most patients are diagnosed at an advanced stage. Targeting tumor-associated antigens PD-1 and PD-L1, VEGF and PARP inhibitors have been highly successful in immunotherapy for certain cancers, but are limited in some cancers, such as ovarian and pancreatic cancers. Therefore, improving efficacy and reducing resistance are of clinical importance in ovarian cancer treatment.
In ovarian cancer OVCAR-5 cell line, high expression of ZG16B promoted tumor migration, invasion, and metastatic ability, and induced activation of Src, ERK, and AKT-related signaling pathways. Knockdown of ZG16B dramatically reduced the cell migration and invasion. In a human ovarian cancer model, a newly designed humanized anti-ZG16B antibody significantly restrained the growth of ovarian tumors. In addition, the combination of Docetaxel and anti-ZG16B achieved the remarkable anti-tumor effect among all treatment groups. The result suggests that targeting ZG16B can be used as a sensitizer for cytotoxic antitumor agents [4].
3.3 ZG16B and Breast Cancer
Breast cancer is the most diagnosed cancer in women. According to the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, breast cancers are clinically classified into four subtypes: luminal A (ER/PR, HER2-), luminal B (ER/PR, HER2), HER2 overexpressed (ER-/PR-/HER2), and basal-like (ER-/PR-/HER2-). In previous studies, several biomarkers have been investigated for early diagnosis and prognosis of breast cancer, such as uPA, Rs/DJ-1, and PAI-1. Recently, uPAR, KiSS1, CD24 etc. have also been suggested to be closely associated with the development of breast cancer. Using STRING analysis and Oncomine co-expression analysis, ZG16B was found to correlate with many biomarkers of breast cancer, including S100PBP, FOXA1, ANKEF1, PRR4, ANKEF1, EPCAM, SPDEF, KRT8, KIAA1324, KRT19, KIAA1324, CXCR4, AGR2, TFAP2A, AP1M2, CLDN4, and ERBB3.
Among them, SPINT1, one of the Kunitz-type serine protease inhibitors, also known as HAI-1, inhibits breast cancer migration, proliferation, and invasion by regulating HGFA, matriptase, and hepsin; TFAP2A or AP-2-α, is a transcription factor that could inhibit breast cancer cell invasion by regulating multiple miRNAs to inhibit the cell cycle; high expression of FOXA1 could elevate the epithelial mesenchymal transition process and inhibit cell migration, invasion, and metastasis. Since these genes co-expressed with ZG16B, the interactions suggested that ZG16B may function as a potential biomarker for breast cancer [1].
3.4 ZG16B and Other Tumors
ZG16B has also been reported as a biomarker for early diagnosis and prognosis of colorectal cancer, prostate cancer, and oral squamous cell carcinoma. ZG16B overexpression enhances cell migration and invasion, leading to poor prognosis [1, 14-16]. In addition, ZG16B has been shown to be upregulated in cervical cancer HeLa cells [17-18]. Multiple studies suggested that ZG16B is associated with prognosis in atherosclerosis and acute coronary syndromes [19-20]. ZG16B has also been demonstrated to be abundant in reflex tears and may act as a key factor in ocular surface protection to maintain tear film stability [21-22]. Currently, most studies focused on the important role of ZG16B as a novel biomarker or therapeutic target in tumors.
4. The Clinical Prospect of ZG16B
Presently, four drugs targeting ZG16B are in clinical trials from Prestige BioPharma company. Specifically, Ulenistamab (PBP1510), IDC 002, IDC 001, and IDC 004, are mainly for pancreatic cancer, pancreatic follicular cancer, ovarian cancer, and ovarian epithelial cancer. Among them, Ulenistamab is the first pancreatic cancer upregulation factor monoclonal antibody. Ulenistamab was approved in France in 2021 for phase I/IIa clinical trials for pancreatic cancer, which is currently in clinical phase 2. The others are biospecific antibodies in preclinical stage (Table 1).
Pancreatic cancer is known to be challenging to treat because of the dense fibrotic tissue surrounding the tumor, which acts as a "fortress" and hinders the delivery of chemotherapy drugs. In recent years, with the advancement of molecular targeted immunotherapy, the treatment of pancreatic cancer has advanced from the ineffective chemotherapy and radiotherapy to the more precise and effective targeted immunotherapy. Various new drugs and therapies have challenged the deadly cancer and gained many breakthroughs. ZG16B, as a new target for pancreatic cancer, is expected to provide important insights for pancreatic cancer immunotherapy.
|
Drugs
|
Target
|
Mechanism
|
Indications
|
R&D Status
|
Institutes
|
Drug Type
|
|
Ulenistamab
|
ZG16B
|
PAUF
|
Pancreatic cancer;
Ovarian cancer
|
Clinical Phase 2
|
Prestige BioPharma Ltd.
|
Monoclonal antibodies
|
|
IDC 002
|
ZG16B
|
T-lymphocyte stimulating agents;
ZG16B inhibitors;
Antibody-dependent cytotoxic effects
|
Epithelial carcinoma of the ovary;
Alveolar carcinoma of the pancreas
|
Drug Discovery
|
Prestige BioPharma Ltd.
|
Bispecific antibodies
|
|
IDC 001
|
ZG16B;
CTHRC1
|
ZG16B inhibitor;
CTHRC1 modulator
|
Ovarian epithelial carcinoma;
Pancreatic cancer
|
Drug Discovery
|
Prestige BioPharma Ltd.
|
Bispecific antibodies
|
|
IDC 004
|
VEGFA;
ZG16B
|
VEGFA inhibitors;
ZG16B inhibitors;
Angiogenesis inhibitors
|
Ovarian epithelial carcinoma;
Pancreatic cancer
|
Drug Discovery
|
Prestige BioPharma Ltd.
|
Bispecific antibodies
|
Table 1: Progress of ZG16B clinical studies
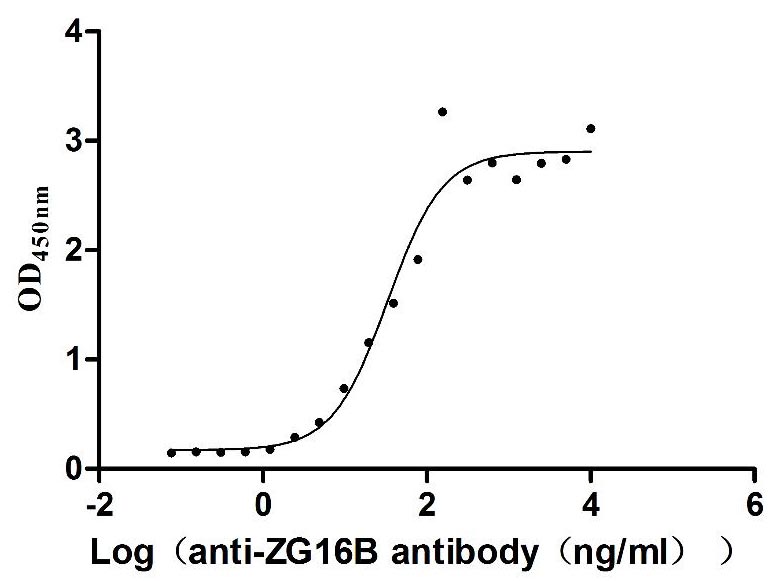
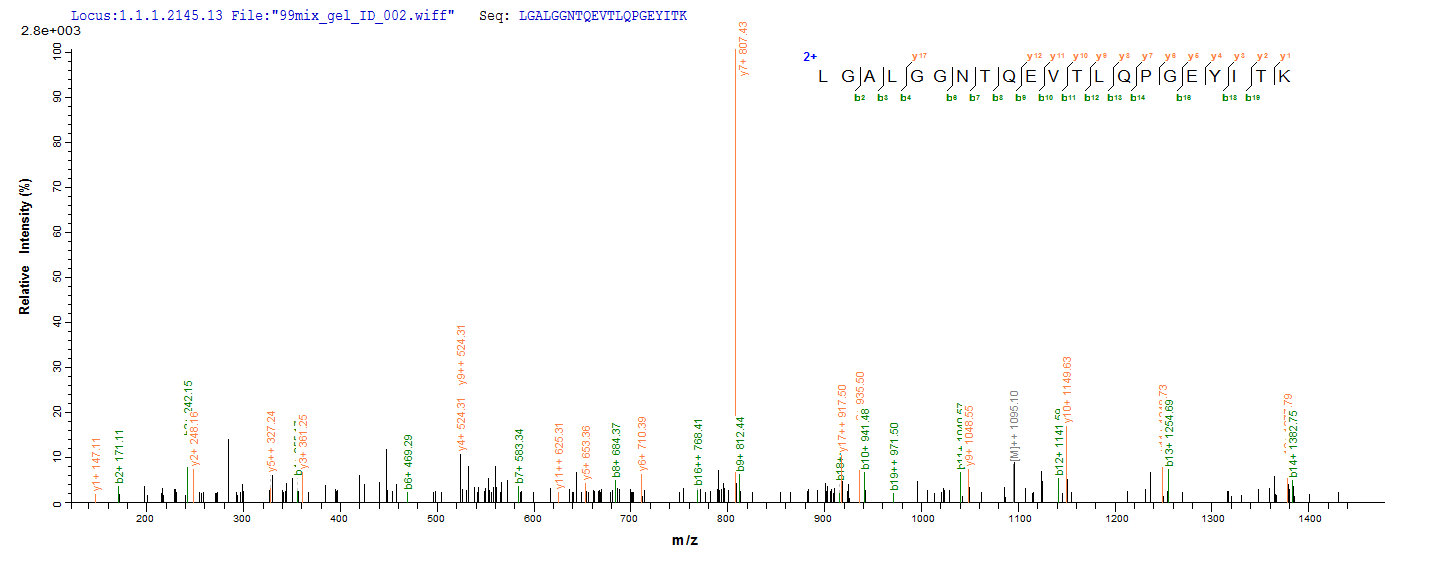
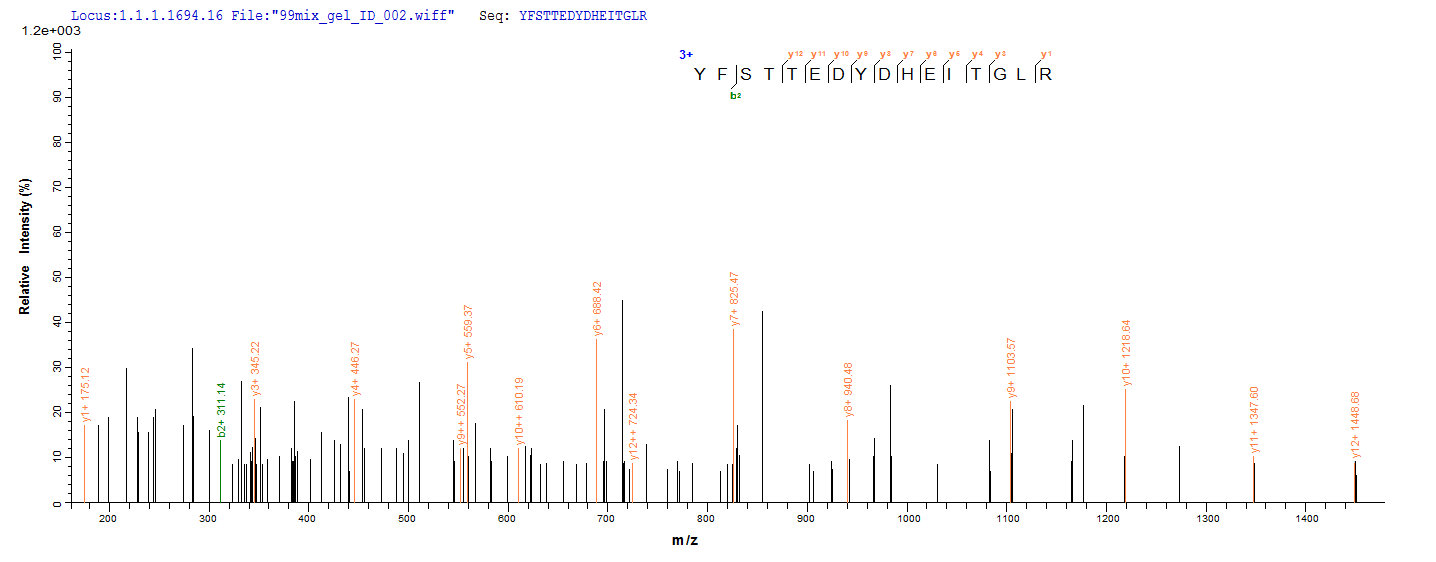
To fully support researchers and pharmaceutical companies in their research on ZG16B based drug research in pancreatic cancer and other diseases, CUSABIO presents ZG16B protein products (CSB-MP836195HU; CSB-YP836195HU; CSB-EP836195HU) to assist you in your research on ZG16B mechanism or its potential clinical value.
● High Purity Validated by Western Blot
● Excellent Bioactivity Validated by Functional ELISA & Protein Sequence Validated by LC-MS/MS Analysis
References
[1] Lu, Haotian, et al. "Identification of ZG16B as a prognostic biomarker in breast cancer." Open Medicine 16.1 (2020): 1-13.
[2] Kanagawa, Mayumi, et al. "Crystal structures of human secretory proteins ZG16p and ZG16b reveal a Jacalin-related β-prism fold." Biochemical and biophysical research communications 404.1 (2011): 201-205.
[3] Kim, Sun A., et al. "Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. "Cancer science 100.5 (2009): 828-836.
[4] Kim, Yeon Jeong, et al. "PAUF as a Target for Treatment of High PAUF-Expressing Ovarian Cancer." Frontiers in Pharmacology 13 (2022).
[5] Patidar, Ashok, et al. "DAMP-TLR-cytokine axis dictates the fate of tumor." Cytokine 104 (2018): 114-123.
[6] Song, Jinhoi, et al. "Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer." Oncotarget 7.32 (2016): 51840.
[7] Li, Jing, et al. "The role of toll-like receptor 4 in tumor microenvironment." oncotarget 8.39 (2017): 66656.
[8] Lee, Y., et al. "PAUF functions in the metastasis of human pancreatic cancer cells and upregulates CXCR4 expression." Oncogene 29.1 (2010): 56-67.
[9] Escudero-Paniagua, Beatriz, et al. "PAUF/ZG16B promotes colorectal cancer progression through alterations of the mitotic functions and the Wnt/β- catenin pathway." Carcinogenesis 41.2 (2020): 203-213.
[10] Yanai, Hideyuki, Sho Hangai, and Tadatsugu Taniguchi. "Damage-associated molecular patterns and Toll-like receptors in the tumor immune microenvironment." International Immunology 33.12 (2021): 841-846.
[11] Dong, Liangchao, Weiwei Li, and Xiaoli Zhang. "Knockdown of pancreatic adenocarcinoma upregulated factor (PAUF) suppresses proliferation, migration, invasion, and cancer stem cell properties in lung cancer cells." Tropical Journal of Pharmaceutical Research 20.3 (2021): 459-465.
[12] Mandakhalikar, Kedar Diwakar, et al. "First-in-class monoclonal antibody (mAb) PBP1510 targeting pancreatic adenocarcinoma upregulated factor ( PAUF) for pancreatic cancer (PC) treatment: Preclinical perspectives." (2022): e16274-e16274.
[13] Kang, Byung Woog, et al. "Genetic variations in miRNA binding site of TPST1 and ZG16B associated with prognosis for patients with colorectal cancer." ( 2013): 3553-3553.
[14] Jin, Hong-Jian, et al. "Identification and validation of regulatory SNPs that modulate transcription factor chromatin binding and gene expression in prostate cancer." Oncotarget 7.34 (2016): 54616.
[15] Chen, Wei, et al. "Identification of core biomarkers associated with pathogenesis and prognostic outcomes of laryngeal squamous-cell cancer using bioinformatics analysis." European Archives of Oto-Rhino-Laryngology 277.5 (2020): 1397-1408.
[16] Barderas, Rodrigo, et al. "In-depth characterization of the secretome of colorectal cancer metastatic cells identifies key proteins in cell adhesion, migration, and invasion." Molecular & Cellular Proteomics 12.6 (2013): 1602-1620.
[17] Kim, Jihye, et al. "Genomic network-based analysis reveals pancreatic adenocarcinoma up-regulating factor-related prognostic markers in cervical carcinoma." Frontiers in oncology 8 (2018): 465.
[18] Sasahira, Tomonori, et al. "Pancreatic adenocarcinoma up-regulated factor has oncogenic functions in oral squamous cell carcinoma." Histopathology 70.4 (2017): 539-548.
[19] Martin-Lorenzo, Marta, et al. "KLK1 and ZG16B proteins and arginine-proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery." Metabolomics 11.5 (2015): 1056-1067.
[20] Rollefstad, S., et al. "Rosuvastatin induced carotid plaque regression in patients with inflammatory joint diseases." atherosclerosis 235.2 (2014): e93.
[21] Costa-da-Silva, Ana Caroline, et al. "Salivary ZG16B Expression Loss Marks Onset of Oral Chronic Graft-Versus-Host Disease and Exocrine Gland Dysfunction." Available at SSRN 3906182.
[22] Ma, Jessica Yuen Wuen, et al. "Critical role of mass spectrometry proteomics in tear biomarker discovery for multifactorial ocular diseases." International Journal of Molecular Medicine 47.5 (2021): 1-15.
[23] Kanagawa, Mayumi, et al. "Crystal structures of human secretory proteins ZG16p and ZG16b reveal a Jacalin-related β-prism fold." Biochemical and biophysical research communications 404.1 (2011): 201-205.
CUSABIO team. ZG16B: A New Tumor Biomarker Offers Important Insights for Drug Development in Pancreatic Cancer!. https://www.cusabio.com/c-21079.html





Comments
Leave a Comment