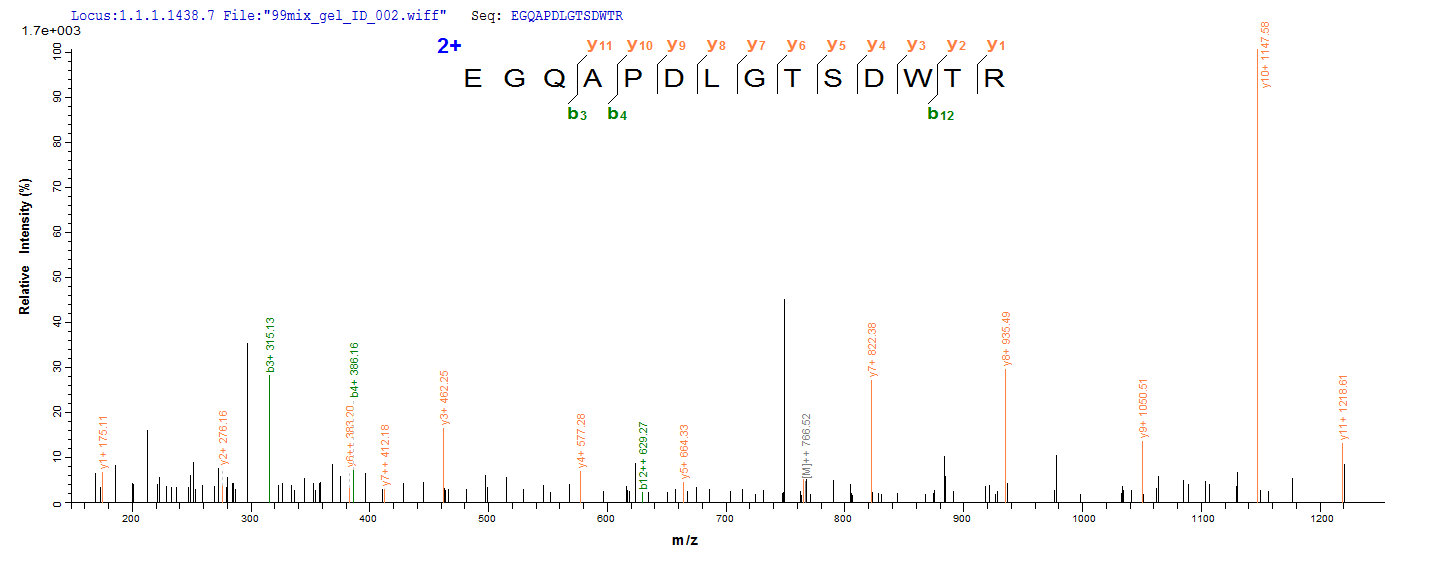
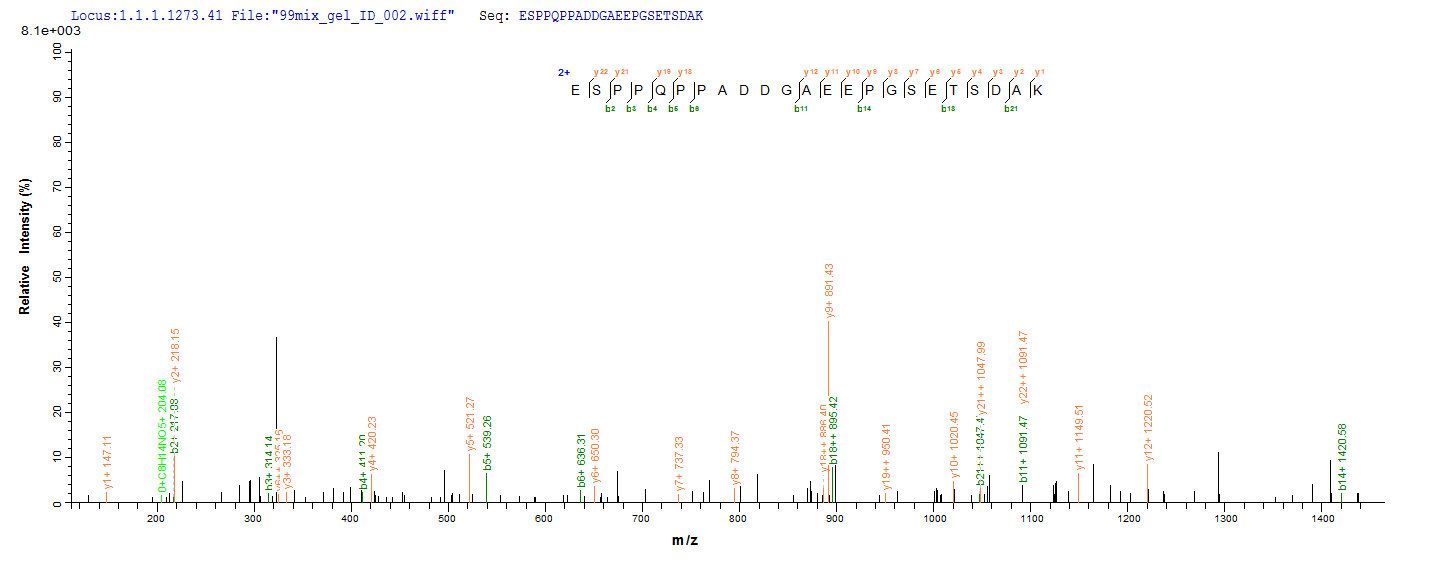
Recombinant full-length of mature Mouse Microtubule-associated protein tau (Mapt) cDNA (2-733aa) constructed with a 6xHis-tag at the N-terminus was expressed in the yeast. The protein identity was confirmed by both SDS-PAGE and LC-MS/MS analysis. It is over 90% in purity and has a calculated molecular weight of 78.1 kDa. Importantly, this recombinant Mapt protein is in stock so there is no waiting period for product preparation. The Mapt can act as an immunogen to elicit an adaptive immune reaction and thus obtain specific antibodies. Besides, this protein may also be used in the studies of the neuroscience field.
Mapt is abundant in the axons of neurons where it promotes microtubule (MT) assembly and stability. Together with stathmin and other destabilizing MAPs, Mapt also participates in MT dynamics via the modulation of assembly, dynamic behavior, and the spatial construction of MTs. Aberrant hyperphosphorylation of Mapt-causing its self-aggregation into paired helical filaments and buildup in the neurons frequently occurred in the tauopathies such as Alzheimer's disease (AD) and frontotemporal dementia (FTD).