[1] Oh, E. S., and Rabins, P. V. (2019). Dementia [J]. Ann. Intern. Med. 171, ITC33–ITC48.
[2] Lyketsos, C. G., Carrillo, et al. (2011). Neuropsychiatric symptoms in Alzheimer's disease [J]. Alzheimer's Dement. 7, 532–539.
[3] Bird, T. D. Alzheimer disease overview. GeneReviews®[Internet] (2018).
[4] Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing and function [J]. J Biol Chem. 2008;283(44):29615–29619.
[5] Querfurth H W, Laferla F M. Alzheimer's disease [J]. N Engl J Med, 2010, 362(4):329-344.
[6] Livingston G, Sommerlad A, Orgeta V,et al. Dementia prevention, intervention, and care [J]. Lancet, 2017, 390(10113).
[7] Livingston, G., Huntley, J., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission [J]. Lancet 396, 413–446.
[8] Harvey R, Skelton-Robinson M, Rossor M. The prevalence and causes of dementia in people under the age of 65 years [J]. Journal of Neurology Neurosurgery & Psychiatry, 2003, 74(9):1206.
[9] Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease [J]. Nature Reviews Neurology, 2011, 7(3):137-152.
[10] Chan K Y, Wang W, Wu J J, et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990–2010: a systematic review and analysis [J]. The Lancet,2013, 381(9882): 2016-2023.
[11] Savage M J, Lin Y G, Ciallella J R, et al. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer's disease model is associated with amyloid deposition [J]. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 2002, 22(9):3376.
[12] Shoji M, Iwakami N, Takeuchi S, et al. JNK activation is associated with intracellular beta-amyloid accumulation [J]. Molecular Brain Research, 2000, 85(1-2):221-233.
[13] CristianaAtzori, BernardinoGhetti, RobertoPiva, et al. Activation of the JNK/p38 Pathway Occurs in Diseases Characterized by Tau Protein Pathology and Is Related to Tau Phosphorylation But Not to Apoptosis [J]. J Neuropathol Exp Neurol, 2001, 60(12):1190-1197.
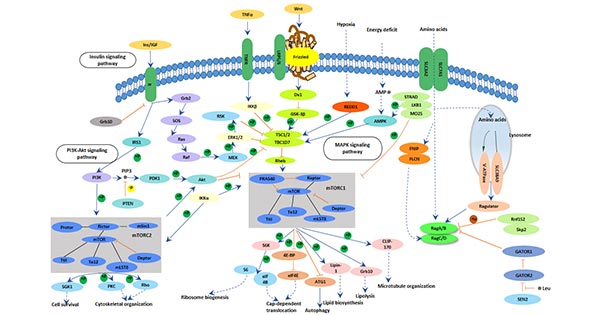
[14] Lafay-Chebassier C, Paccalin M, Page G, et al. mTOR/p70S6k signalling alteration by A-beta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer's disease [J]. Journal of Neurochemistry, 2005, 94(1):215-225.
[15] Grimm MO1, Hundsdörfer B, Grösgen S, et al. PS dependent APP cleavage regulates glucosylceramide synthase and is affected in Alzheimer's disease [J] . Cell Physiol Biochem, 2014, 34: 92-110.
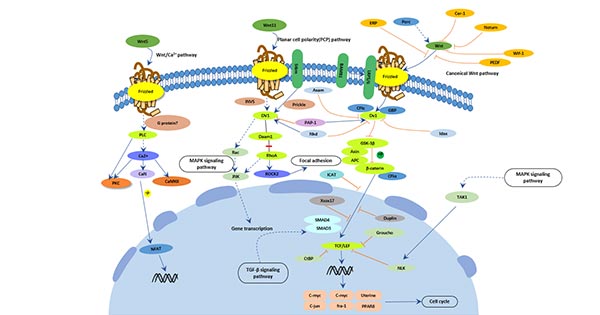
[16] Boonen R A, Van T P, Zivkovic D. Wnt signaling in Alzheimer's disease: up or down, that is the question [J]. Ageing Research Reviews, 2009, 8(2):71-82.
[17] Cheng S., Banerjee S., et al. Novel blood test for early biomarkers of preeclampsia and Alzheimer's disease [J]. Sci. Rep. 2021;11:15934.
[18] Goldoni R., Dolci C., et all. Salivary biomarkers of neurodegenerative and demyelinating diseases and biosensors for their detection. Ageing Res. Rev. 2022;76:101587.
[19] Borhani N., Ghaisari J., Abedi M., Kamali M., Gheisari Y. A deep learning approach to predict inter-omics interactions in multi-layer networks [J]. BMC Bioinform. 2022;23:53.
[20] Meghdadi A.H., Stevanović Karić M., et al. Resting state EEG biomarkers of cognitive decline associated with Alzheimer's disease and mild cognitive impairment [J]. PLoS ONE. 2021;16:e0244180.
[21] Eyigoz E., Mathur S., et al. Linguistic markers predict onset of Alzheimer's disease [J]. EClinicalMedicine. 2020;28:100583.
[22] Gunes S, Aizawa Y, et al. Biomarkers for Alzheimer's Disease in the Current State: A Narrative Review [J]. Int J Mol Sci. 2022 Apr 29;23(9):4962.
[23] Silvestro S, Bramanti P, Mazzon E. Role of miRNAs in Alzheimer's disease and possible fields of application [J]. Int J Mol Sci. 2019;20(16):3979.
[24] Reddy PH, Tonk S, et al. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer's disease [J]. Biochem Biophys Res Commun. 2017;483(4):1156–65.

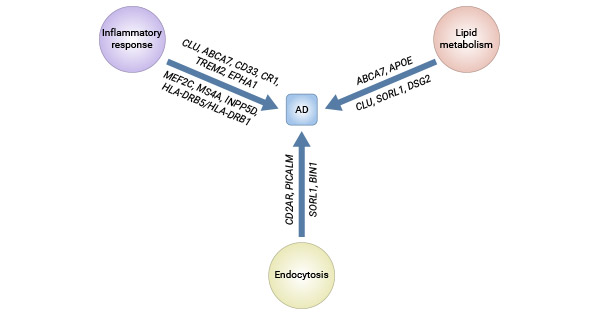
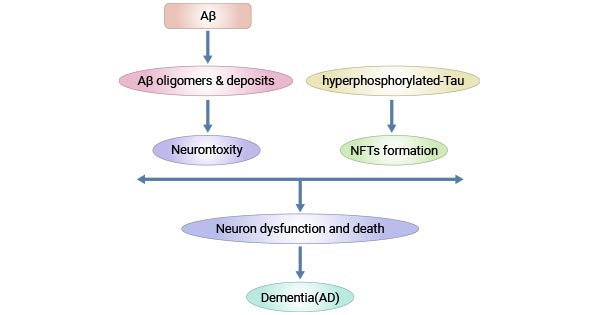
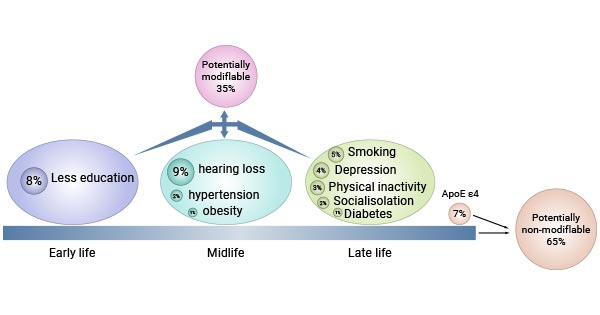
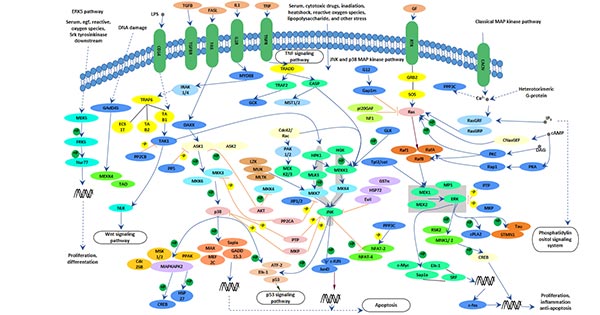


Comments
Leave a Comment