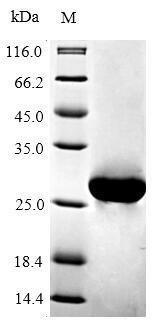
The gene fragment encoding the 5-205aa of the mouse BCL2 is co-expressed with the N-terminal 6xHis-tag gene in E.coli. The resulting product is the recombinant mouse BCL2, which is purified through affinity chromatography. Its purity is over 90%, confirmed by SDS-PAGE. Available in liquid or lyophilized powder, this recombinant BCL2 is perfect for experiments focusing on apoptosis, providing researchers with reliable and consistent results.
Mouse BCL2 is crucial in regulating apoptosis, particularly in B lymphocytes. In mice, BCL2 is expressed at various stages of B cell development, particularly in pro- and pre-B cells, and is crucial for early B lymphopoiesis. However, its expression diminishes in germinal centers, where B cells undergo selection and differentiation [1].
Research has demonstrated that transgenic mice overexpressing BCL2 exhibit enhanced survival rates and reduced apoptosis in various contexts, including models of sepsis and ischemia-reperfusion injury [2][3]. Furthermore, studies involving BCL2 transgenic mice have revealed that these mice have a predisposition to develop lymphomas, particularly follicular lymphoma, due to the extended lifespan of B cells and the inhibition of apoptosis [4].
Beyond lymphocyte survival, BCL2 is also implicated in bone metabolism. In osteoblasts, BCL2 overexpression can inhibit differentiation and promote apoptosis of osteocytes, indicating a complex relationship between BCL2 and bone health [5]. Additionally, the BCL2/BAX ratio is a critical determinant of cell fate, where an increase in BCL2 levels can lead to enhanced cell proliferation, while elevated BAX levels promote apoptosis [6]. This balance is essential for maintaining homeostasis in various tissues, including bone and lymphoid tissues.
References:
[1] C. Brunner, D. Marinković, J. Klein, T. Samardžić, L. Nitschke, & T. Wirth, B cell–specific transgenic expression of bcl2 rescues early b lymphopoiesis but not b cell responses in bob.1/obf.1-deficient mice, The Journal of Experimental Medicine, vol. 197, no. 9, p. 1205-1211, 2003. https://doi.org/10.1084/jem.20022014
[2] A. Iwata, V. Morgan-Stevenson, B. Schwartz, L. Liu, J. Tupper, X. Zhuet al., Extracellular bcl2 proteins are danger-associated molecular patterns that reduce tissue damage in murine models of ischemia-reperfusion injury, Plos One, vol. 5, no. 2, p. e9103, 2010. https://doi.org/10.1371/journal.pone.0009103
[3] A. Iwata, R. Claro, V. Morgan-Stevenson, J. Tupper, B. Schwartz, L. Liuet al., Extracellular administration of bcl2 protein reduces apoptosis and improves survival in a murine model of sepsis, Plos One, vol. 6, no. 2, p. e14729, 2011. https://doi.org/10.1371/journal.pone.0014729
[4] A. Egle, A. Harris, M. Bath, L. O’Reilly, & S. Cory, Vavp-bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia, Blood, vol. 103, no. 6, p. 2276-2283, 2004. https://doi.org/10.1182/blood-2003-07-2469
[5] T. Moriishi, Z. Maruyama, R. Fukuyama, M. Ito, T. Miyazaki, H. Kitauraet al., Overexpression of bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosis, Plos One, vol. 6, no. 11, p. e27487, 2011. https://doi.org/10.1371/journal.pone.0027487
[6] J. Oh, J. Lee, J. Park, J. No, & N. Lee, Obatoclax regulates the proliferation and fusion of osteoclast precursors through the inhibition of erk activation by rankl, Molecules and Cells, vol. 38, no. 3, p. 279-284, 2015. https://doi.org/10.14348/molcells.2015.2340