TLRs are important receptors involved in innate immunity, and they can specifically identify and combine pathogen-associated molecular patterns (PAMPs), triggering a series of signal transduction, which leads to the release of inflammatory mediators, eventually activates the adaptive immune system [4] [12].
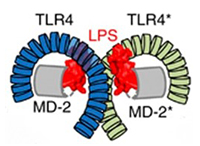
Bruce A. Beutler' group demonstrated that TLR4 is an important sensor for LPS by using the C3H/HeJ mouse strain which is known to have a hypoergia to LPS [5]. TLR4 can recognize LPS and thereby release a series of cytokines that promote proinflammatory response [27]. TLR4 is also known as an amplifier for the inflammatory reaction [6] [7].
4. Recent Research on TLR4
On May 7, 2019, a study online published in journal Cell Reports, described that Cincinnati Children's scientists have discovered that one of the major risk factors of preterm birth originates from TLR4 in the endothelial cells in the lining of blood vessels of the decidua. And the findings may provide a potential therapeutic target for preventing preterm birth.
To mimic how infections trigger inflammation and further lead to preterm birth, the researchers used LPS to stimulate pregnant mice. They found that the mice response to LPS begins with the activation of TLR4 present on decidual vascular endothelial cells.
Activated TLR4 by LPS then promotes the release of a series of proinflammatory cytokines, including IL6. This process helps the body fight against infections. As IL6 elevates, IL10 releases to recover the inflammatory level. It keeps a balanced immunity. However, once TLR4 is excessively activated, IL10 cannot be enough to respond to restore the inflammatory level, which breaks the balanced immunity, causing a dangerous inflammation and a higher risk of preterm birth.
The team knocked out TLR4 in mice' decidual tissue and found that mice are highly sensitive to LPS, even at low doses, and are likely to experience preterm birth. And managing IL10 helps these mice avoid premature birth. However, deletion of TLR4 in vascular endothelial cells renders mice highly resistant to LPS-induced preterm birth, even at high doses.
These results indicate that systemic inflammation begins with TLR4 in decidual vascular endothelial cells, which is one of the high risks leading to preterm birth.
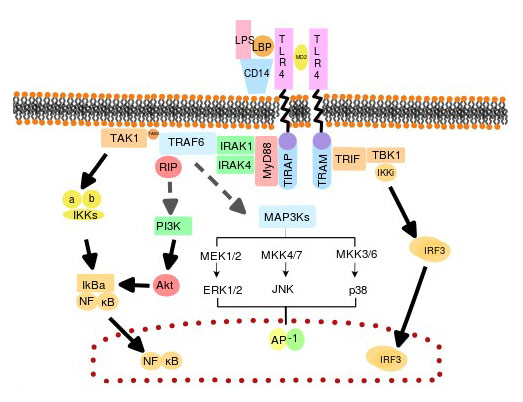
In conclusion, TLR4 is a peculiar member of the TLR family. And it is the only known TLR which uses all those adaptor proteins. TLR4 can specifically recognize and bind to LPS, eventually triggering a proinflammatory cascade. And the LPS/TLR4 signal transduction pathway is involved in many diseases, which potentially provides strategies to study and develop therapeutic ways or drugs for these related diseases.
References
[1] Hansson GK, Edfeldt K, et al. Toll to be paid at the gateway to the vessel wall [J]. Arterioscler Thromb. Vasc. Biol. 2005, 25 (6): 1085–7.
[2] Hashimoto C, Hudson KL, et al. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein [J]. Cell. 1988, 52 (2): 269–79.
[3] Medzhitov R, Preston-Hurlburt P, et al. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity [J]. Nature. 1997, 388 (6640): 394–7.
[4] Fitzgerald, K. et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF [J]. J Exp Med. 2003, 198, 1043-1055.
[5] Medzhitov, R., Preston-Hurlburt, et al. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity [J]. Nature 388. 1997, 394-397.
[6] Hoshino, K, Takeuchi, O, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product [J]. Journal of Immunology. 1999, 162 (7): 3749–52.
[7] Poltorak, Alexander, et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene [J]. Science. 1998, 282 (5396): 2085–2088. .
[8]?Vaure C, Liu Y,?A comparative review of toll-like receptor 4 expression and functionality in different animal species [J].?Frontiers in Immunology. 2014,?5: 316.?
[9] Lu YC, Yeh WC, et al. LPS/TLR4 signal transduction pathway.?Cytokine. 2008,?42?(2): 145–51.
[10] P?lsson-McDermott EM, O'Neill LA.?Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4 [J].?Immunology. 2004,?113?(2): 153–62.
[11] O'Neill LA, Golenbock D, et al. The history of Toll-like receptors-redefining innate immunity [J].?Nature Reviews. Immunology. 2013,?13?(6): 453–60.?/p>
[12] Kawai, T. Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors [J].?Nat Immunol.2010,?11(5): 373–384.
[13] Triantafilou, M., K, et al. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster [J].?Trends Immunol. 2002, 23(6):301-4.
[14] da Silva Correia, J., K. Soldau, et al. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex: transfer from CD14 to TLR4 and MD-2 [J].?J Biol Chem. 2001,?276(24):21129-35.
[15] Miyake, K., Innate recognition of lipopolysaccharide by CD14 and Toll-like receptor 4-MD-2: unique roles for MD-2 [J].?Int Immunopharmacol. 2003,?3(1):119-28.
[16] Slack, J. L., K. Schooley, et al. Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways [J].?J Biol Chem. 2000,?275(7):4670-8.
[17] Jiang, Z., M. Zamanian-Daryoush, et al. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFκB and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR [J].?J Biol Chem. 2003,?278(19):16713-9.
[18] Ghosh, S., M. Karin. Missing pieces in the NF-κB puzzle.?Cell. 2002,?109 Suppl:S81-96.
[19] Fitzgerald, KA, Palsson-McDermott EM, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction [J].?Nature 2001,?413(6851):78-83.
[20] Toshchakov V, Jones BW, et al TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages [J].?Nat Immunol.?3(8):392-8.
[21] Yamamoto, M., S. Sato, et al. Cutting Edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling [J].?J Immunol. 2002,?169(12):6668-72.
[22] Salkowski, C. A., K. E. Thomas, et al.?Impaired IFN-γ production in IFN regulatory factor-1 knockout mice during endotoxemia is secondary to a loss of both IL-12 and IL-12 receptor expression [J].?J Immunol. 2000,?165(7):3970-7.
[23] Scanzello, CR,?Plaas A, et al.?Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound [J]? Curr Opin Rheumatol. 2008,?20(5):565–572.
[24] Steven R Goldring?&?Carla R Scanzello.?Plasma proteins take their toll on the joint in osteoarthritis [J].?Arthritis Research & Therapy, 2012,?14:111.
[25] Kikuchi T, Matsuguchi T,?et al.?Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors [J].?J. Immunol. 2001, 166(5):3574-9.
[26] Sohn, D. H.?et al.?Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4 [J].?Arthritis Research therapy, 2012, Ther.?14(1):R7.
[27] Lien, E, Means TK,?et al.?Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide [J].?J Clin Invest. 2000,?105(4):497–504.
[28] Hakim F, Wang Y, et al Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling [J].?Cancer Res.?2014, 749(5):1329–37.
[29] Lissbrant IF, Stattin P, et al. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival [J].?Int J Oncol.?2000, 17(3):445–51.
[30] Koukourakis MI, Giatromanolaki A, et al. Different patterns of stromal and cancer cell thymidine phosphorylase reactivity in non-small-cell lung cancer: impact on tumour neoangiogenesis and survival [J].?Br J Cancer.?1998, 77(10):1696–703.
[31] Farina C, Aloisi F, et al.?Astrocytes are active players in cerebral innate immunity [J].?Trends Immunol.?2007,?28(3):138-45.




Comments
Leave a Comment