Song Wei's team reveals the pathogenesis of cancer cachexia: gut-kidney immune axis and uric acid metabolism
Cancer cachexia or tumor-induced host wasting is common in a variety of cancers, such as pancreatic cancer, gastric cancer, lung cancer, colorectal cancer, etc. It is one of the important causes of death in cancer patients. More than 60% of cancer patients are affected by cachexia. Cancer cachexia is accompanied by persistent weight loss, hyperglycemia, and high mortality from patient muscle and adipose tissue (Argiles et al., 2014). Because of its unknown pathogenesis and lack of effective treatments, cancer cachexia has also been rated as "the last human disease" (Lok, 2015).
Using different mouse tumor models in the past, researchers have found that malignant tumors can promote host depletion by secreting proteins, such as IL-6, TNF-α, Activin A, LIF, etc. (Baracos et al., 2018). However, these studies are usually done under specific pathogen-free (SPF) conditions, thus ignoring the role of microbes in the environment such as bacteria, fungi, viruses, etc. and the host immune response.
However, the microbe-host interaction is involved in various physiological processes and the occurrence of various diseases in the host body (Cani, 2017). Unlike the mouse model, the flies were in direct contact with the outside air and microbes during the study. And as an evolutionarily conserved model organism, Drosophila has gradually become an ideal model for studying tumor-induced host depletion in recent years. The phenotypes of cancer cachexia, such as fat loss and muscle wasting, hyperglycemia, and high mortality, can be completely mimicked by inducing malignant tumors in Drosophila or transplanting exogenous tumors (Figueroa-Clarevega and Bilder, 2015; Kwon et al., 2015).
Using the Drosophila cachexia model, the researchers identified a series of tumor-secreted proteins, such as ImpL2, Pvf1 and Upd3, which remotely disrupt host organ metabolic balance through insulin, MEK and Jak/Stat signaling pathways, respectively, resulting in host depletion (Ding et al., 2021; Kwon et al., 2015; Lodge et al., 2021; Song et al., 2019).
On August 26, 2022, Song Wei's research group from Wuhan University Frontier Science Center for Immunology and Metabolism/Central South Hospital Medical Research Institute/Taikang Life Medical Center published an article entitled "Renal NF-κB activation impairs uric acid homeostasis to promote tumor-associated mortality independent of wasting" in the journal, Immunity.
In this study, the overexpression of the activated transcription factor yki3SA (homolog of human YAP1) in the Drosophila gut induces the excessive proliferation of intestinal stem cells to form intestinal malignancies. In this study, the researchers first found that yki3SA intestinal tumor flies had an exponential increase in bacteria and fungi compared to controls, while exhibiting hyperactivation of systemic immunity, mainly IMD, which mediates immune responses to Gram-negative bacteria -NF-κB, but not Toll-NF-κB signaling activation. Using antibiotic treatment and germ-free Drosophila methods to inhibit bacterial proliferation and systemic IMD-NF-κB activation in yki3SA Drosophila, prolong the lifespan of tumor-bearing Drosophila. Genetically restoring intestinal PGRP-SC2 (identified in this study as a novel secreted amidase with broad-spectrum antibacterial function) expression also inhibited bacterial proliferation and IMD- NF-κB activation extends lifespan in Drosophila.
The researchers further found that only inhibition of the IMD-NF-κB pathway in the Malpighian tube of yki3SA flies, but not muscle, fat, and brain tissue traditionally thought, could effectively alleviate the mortality of yki3SA tumor-bearing flies. Combined with transcriptomic, metabolomic and single-cell sequencing analysis, it was found that IMD-NF-κB activation in Malpighian tube of yki3SA tumor-bearing Drosophila can cause uric acid accumulation and promote body death; feeding Allopurinol inhibits uric acid synthesis Or specifically blocking the IMD-NF-κB pathway in Malpighian ducts can effectively alleviate the accumulation of uric acid in yki3SA tumor-bearing flies and prolong lifespan. Previously, the research group found that ImpL2, an important tumor-secreted protein, can also lead to death in part through renal uric acid metabolism (Figure 1). Importantly, large population-based cohort studies have also found that hyperuricemia (>400 mM) is a significant independent risk factor for mortality in patients with multiple cancers (Shin et al., 2006; Strasak et al., 2007).
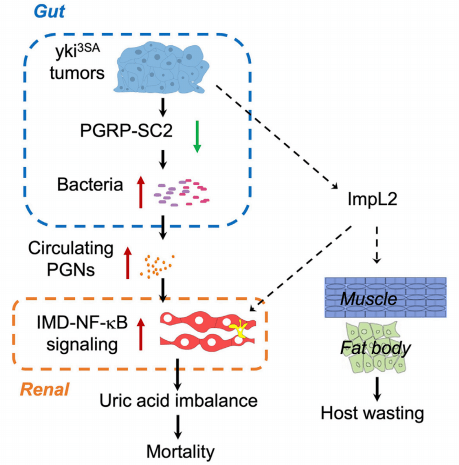
Figure 1
The study found that environmental microbes, gut bacteria, renal IMD-NF-κB immune response, and uric acid metabolism are important factors in malignancy-induced death, independent of currently known tumor-related body consumption. This provides a new perspective for an in-depth understanding of tumor-host interactions and the realization of tumor-bearing survival.
By Yuchen Chen
one of the authors of the article, Renal NF-κB activation impairs uric acid homeostasis to promote tumor-associated mortality independent of wasting
CUSABIO Recombinant PGRP-SC2 protein (Code: CSB-EP892684DLU) was cited in the article.
Reference
Argiles, J.M., Busquets, S., Stemmler, B., and Lopez-Soriano, F.J. (2014). Cancer cachexia: understanding the molecular basis. Nature reviews Cancer 14, 754-762.
Baracos, V.E., Martin, L., Korc, M., Guttridge, D.C., and Fearon, K.C.H. (2018). Cancer-associated cachexia. Nat Rev Dis Primers 4, 17105.
Cani, P.D. (2017). Gut microbiota - at the intersection of everything? Nat Rev Gastroenterol Hepatol 14, 321-322.
Ding, G., Xiang, X., Hu, Y., Xiao, G., Chen, Y., Binari, R., Comjean, A., Li, J., Rushworth, E., Fu, Z., et al. (2021). Coordination of tumor growth and host wasting by tumor-derived Upd3. Cell reports 36.
Figueroa-Clarevega, A., and Bilder, D. (2015). Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell 33, 47-55.
Kwon, Y., Song, W., Droujinine, Ilia A., Hu, Y., Asara, John M., and Perrimon, N. (2015). Systemic Organ Wasting Induced by Localized Expression of the Secreted Insulin/IGF Antagonist ImpL2. Developmental Cell 33, 36-46.
Lodge, W., Zavortink, M., Golenkina, S., Froldi, F., Dark, C., Cheung, S., Parker, B.L., Blazev, R., Bakopoulos, D., Christie, E.L., et al. (2021). Tumor-derived MMPs regulate cachexia in a Drosophila cancer model. Dev Cell 56, 2664-2680 e2666.
Lok, C. (2015). Cachexia: The last illness. Nature 528, 182-183.
Shin, H.S., Lee, H.R., Lee, D.C., Shim, J.Y., Cho, K.H., and Suh, S.Y. (2006). Uric acid as a prognostic factor for survival time: a prospective cohort study of terminally ill cancer patients. J Pain Symptom Manage 31, 493-501.
Song, W., Kir, S., Hong, S., Hu, Y., Wang, X., Binari, R., Tang, H.W., Chung, V., Banks, A.S., Spiegelman, B., et al. (2019). Tumor-Derived Ligands Trigger Tumor Growth and Host Wasting via Differential MEK Activation. Dev Cell 48, 277-286 e276.
Strasak, A.M., Rapp, K., Hilbe, W., Oberaigner, W., Ruttmann, E., Concin, H., Diem, G., Pfeiffer, K.P., Ulmer, H., Vhm, et al. (2007). Serum uric acid and risk of cancer mortality in a large prospective male cohort. Cancer Causes Control 18, 1021-1029.
Cite this article
CUSABIO team. Song Wei's team reveals the pathogenesis of cancer cachexia: gut-kidney immune axis and uric acid metabolism. https://www.cusabio.com/c-21088.html






Comments
Leave a Comment