Monkeypox Virus Research
Related Products
The WHO Director-General announced on 7 August 2024 that he had triggered the process for EUL of mpox vaccines given worrying trends in the disease's spread. There is a serious and growing outbreak in the Democratic Republic of the Congo (DRC) that has now expanded outside the country [1].
Since May 2022, monkeypox outbreaks have appeared in many countries in Europe and the United States, and have rapidly spread to more than 100 countries, once becoming "public health emergencies of international concern", and the WHO has convened a number of International Health Regulations (2005) Committee on Emergencies (CHR) on the monkeypox outbreaks in several countries and issued the "Strategic framework for enhancing prevention and control of mpox (2024–2027) on May 24, 2024 [2].
1. What is Monkeypox?
Monkeypox (Mpox) is a zoonotic disease caused by infection with the monkeypox virus (MPXV), characterized by fever, rash, and enlarged lymph nodes. Monkeypox virus was first identified in monkeys in 1958, and the first confirmed human case was found in the Democratic Republic of Congo in 1970.
Early symptoms of monkeypox infection include non-specific symptoms such as painful fever and enlarged lymph nodes, which are more likely to progress to severe disease in populations including children, the elderly and immunodeficient patients.
Monkeypox can be transmitted through contact with diseased skin, mucous membranes, contaminated objects, or respiratory droplets from cases of prolonged close inhalation, as well as through contact with respiratory secretions, exudates, blood and other body fluids of infected animals, or through bites or scratches from infected animals. Because of its long incubation period, it can lead to widespread transmission among people.
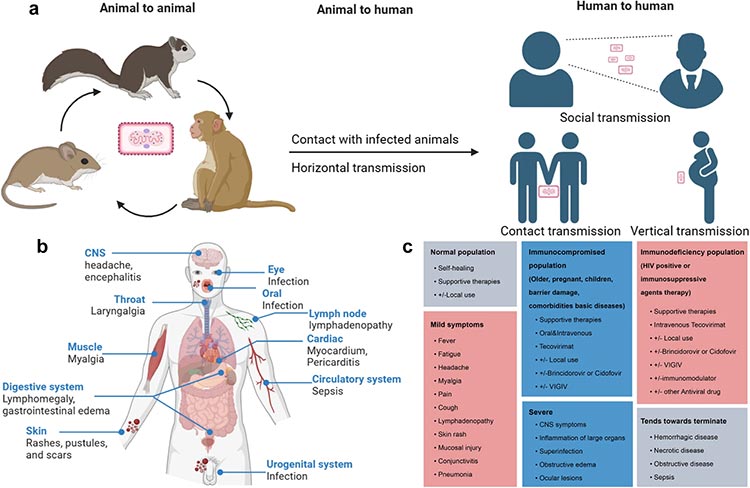
Fig 1. The epidemiological characteristics, pathogenesis, clinical diagnosis, and treatment of Mpox [3]
2. What is Monkeypox Virus?
Monkeypox virus belongs to the genus Orthopoxvirus in the family Poxviridae and is a virus with linear double-stranded DNA. It shares more than 90% sequence similarity with other orthopoxviruses in the same genus in the central core region of the genome, which allows a certain degree of cross-immunization protection for people who have been previously vaccinated against smallpox. However, the specificity of monkeypox virus is mainly in the variable regions at the end of its genome, and differences in these regions have important implications for virulence, host recognition, and immune escape.
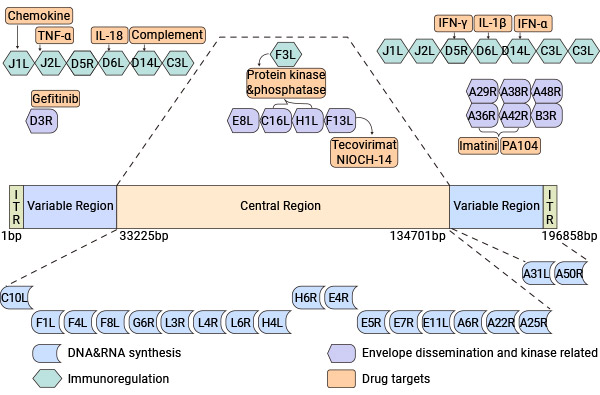
Fig 2. The genome structure and potential antiviral targets of Mpox virus [3]
As of July 30, 2024, 7,719 monkeypox virus genome sequences have been submitted worldwide, mainly in the United States, Germany, Portugal, and the United Kingdom.
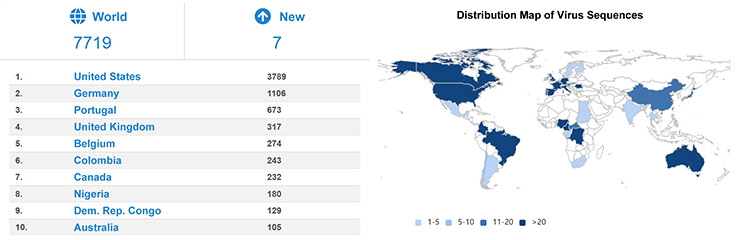
Fig 3. Sequence submissions of monkeypox virus by country [4]
3. How Monkeypox Virus Infection and Replication?
The process of monkeypox virus infection and replication can be summarized in three distinct phases: 1) viral invasion; 2) viral replication and synthesis; and 3) viral assembly, maturation, and release. The virus enters the cell interior by binding to host cell receptors via its surface glycoproteins. Upon completion of viral replication and assembly, new viral particles are released from the host cell by outgrowth, which in turn infects other cells.
A key step in the replication mechanism of monkeypox virus is the synthesis of viral DNA, which requires the involvement of the viral polymerase holoenzyme. The polymerase holoenzyme consists of several key proteins, including F8, A22, E4, and H5, which work together to enhance replication persistence and accuracy.F8 serves as the core catalytic subunit, the A22-E4 dimer enhances the replication persistence of the polymerase, and E4 also possesses uracil DNA glycosidase (UDG) activity, which recognizes and excises uracil monobase mutations in the DNA and initiates the DNA repair pathway [5].
4. What are the Drug Targets for Monkeypox?
Currently, potential antiviral targets of monkeypox virus and drugs are focused on key proteins of the viral replication mechanism, for example:
4.1 Bispecific Phosphatase H1
Monkeypox virus encodes a bispecific phosphatase H1 that inhibits the host interferon signaling pathway, thereby down-regulating the antiviral response of host cells. Resolution of the high-resolution three-dimensional structure of phosphatase H1 revealed the structural basis for its catalytic function and identified two important sites for drug development: the dimer interface of H1 phosphatase and its active center [6].
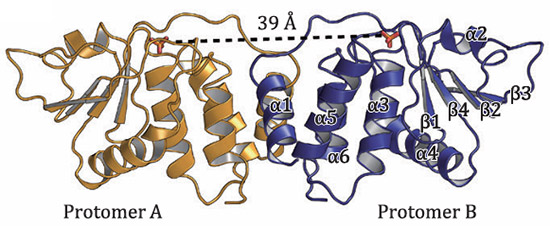
Fig 4. The overall structure of H1 dimer [6]
4.2 Immunomodulatory Protein M2
The M2 protein is an immunomodulatory protein in monkeypox virus that is able to inhibit T cell activation by binding to the B7.1/B7.2 co-stimulatory molecule and thereby antagonizing the CD28/CTLA4-B7.1/2 signaling pathway. This finding provides a new idea for poxvirus defense and reveals the important role of the multimeric form of the M2 protein in viral evasion of T cell immune responses [7].
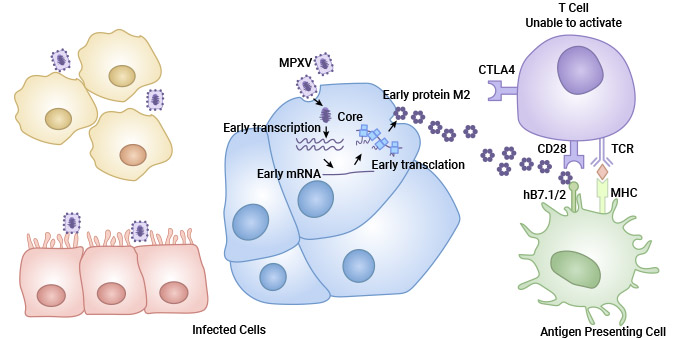
Fig 5. M2blocksTcellactivationmediatedbyhB7.1/2 [7]
4.3 APOBEC3 Protease
APOBEC3 protease is an enzyme within the host cell that acts as a defense against viruses during viral replication and is able to introduce mutations through a deamidation editing mechanism, thereby affecting viral replication and adaptation [8]. This finding is of importance for understanding the mechanisms of adaptation and transmission of monkeypox virus and provides a potential target for the development of antiviral drugs against monkeypox virus.
4.4 Other Orthopoxvirus-related Proteins
In addition to the above targets, the development of small molecule drugs specifically targeting orthopoxvirus-related proteins such as A34R, H3L, A27L, and A28 will also be a direction of development in this field [9].
5. CUSABIO Monkeypox Virus Related Products
5.1 CUSABIO Monkeypox Virus Related Recombinant Proteins
5.2 CUSABIO Monkeypox Virus Research Related Recombinant Proteins
5.3 CUSABIO Monkeypox Virus Research Related Antibodies
| Product name |
Species Reactivity |
Application |
Code |
| A29L Antibody |
Monkeypox virus (strain Zaire-96-I-16) |
ELISA, WB |
CSB-PA46839LA01MPX |
Reference:
[1] WHO invites mpox vaccine manufacturers to submit dossiers for emergency evaluation. (www.who.int/news)
[2] www.who.int/publications/i/item/9789240092907
[3] Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther, 2023.
[4] http://ngdc.cncb.ac.cn/gwh/poxvirus/
[5] Structural insights into the assembly and mechanism of mpox virus DNA polymerase complex F8-A22-E4-H5. Mol Cell, 2023.
[6] Crystal structure of monkeypox H1 phosphatase, an antiviral drug target. Protein Cell, 2023.
[7] Structural and functional insights into the modulation of T cell costimulation by monkeypox virus protein M2. Nat Commun, 2023.
[8] APOBEC3 deaminase editing in mpox virus as evidence for sustained human transmission since at least 2016. Science, 2023.
[9] Human mpox: Biology, epidemiology, therapeutic options, and development of small molecule inhibitors. Med Res Rev, 2023.