[1] Li, Xi, et al. "An immunomodulatory antibody-drug conjugate (ADC) targeting BDCA2 strongly suppresses pDC function and glucocorticoid responsive genes." Rheumatology (Oxford, England) (2023): kead219-kead219.
[2] Wu, Jing, et al. "pDC activation by TLR7/8 ligand CL097 compared to TLR7 ligand IMQ or TLR9 ligand CpG." Journal of Immunology Research 2019 (2019).
[3] Santana-de Anda, Karina, et al. "Plasmacytoid dendritic cells: key players in viral infections and autoimmune diseases." Seminars in arthritis and rheumatism. vol. 43. no. 1. wb Saunders, 2013.
[4] Rogers, N. M., J. S. Isenberg, and A. W. Thomson. "Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance?" American Journal of Transplantation 13.5 (2013): 1125-1133.
[5] Hardy, Andrew W., et al. "HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll- like receptor 7-induced IFN-α." Proceedings of the National Academy of Sciences 104.44 (2007): 17453-17458.
[6] Fitzgerald-Bocarsly, Patricia, and Evan S. Jacobs. "Plasmacytoid dendritic cells in HIV infection: striking a delicate balance." Journal of leukocyte biology 87.4 (2010): 609-620.
[7] Reizis, Boris. "Regulation of plasmacytoid dendritic cell development." Current opinion in immunology 22.2 (2010): 206-211.
[8] Toma-Hirano, Makiko, et al. "Type I interferon regulates pDC maturation and Ly49Q expression." European journal of immunology 37.10 ( 2007): 2707-2714.
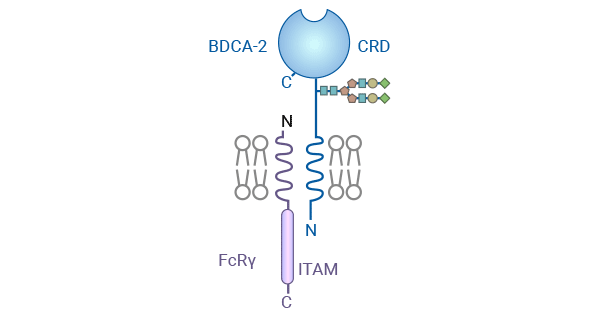
[9] Jégouzo, Sabine AF, et al. "A novel mechanism for binding of galactose-terminated glycans by the C-type carbohydrate recognition domain in blood dendritic cell antigen 2." Journal of Biological Chemistry 290.27 (2015): 16759-16771.
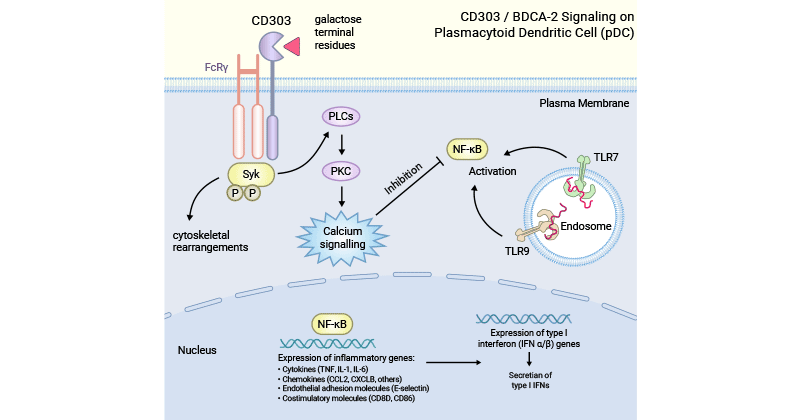
[10] Wilson, Nathaniel R., et al. "CD303 (BDCA-2)-a potential novel target for therapy in hematologic malignancies." Leukemia & lymphoma 63.1 (2022): 19-30.
[11] Furie, Richard A., et al. "Trial of anti-BDCA2 antibody litifilimab for systemic lupus erythematosus." New England Journal of Medicine 387.10 (2022). 894-904.
[12] Furie, Richard, et al. "Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus." The Journal of clinical investigation 129.3 (2019): 1359-1371.
[13] Gardet, Agnes, et al. "Effect of in vivo hydroxychloroquine and ex vivo anti-BDCA2 mAb treatment on pDC IFNα production from patients affected with cutaneous lupus erythematosus." Frontiers in immunology 10 (2019): 275.
[14] Florentin, Jonathan, et al. "HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells." Blood, The Journal of the American Society of Hematology 120.23 (2012): 4544-4551.
[15] Lynch, Jason P., et al. "The plasmacytoid dendritic cell: at the cross-roads in asthma." European Respiratory Journal 43.1 (2014): 264-275.
[16] Van Brussel, Ilse, et al. "Expression of dendritic cell markers CD11c/BDCA-1 and CD123/BDCA-2 in coronary artery disease upon activation in whole blood ." Journal of immunological methods 362.1-2 (2010): 168-175.
[17] Migita, K., et al. "Reduced blood BDCA-2+ (lymphoid) and CD11c+ (myeloid) dendritic cells in systemic lupus erythematosus." Clinical & Experimental Immunology 142.1 (2005): 84-91.
[18] Ibrahim, Hazem Ahmed Hamed. "Pathogenesis of B-cell post-transplant lymphoproliferative disorders and HIV-associated B-cell lymphomas." (2010).
[19] Palma, Giuseppe, et al. "Plasmacytoids dendritic cells are a therapeutic target in anticancer immunity." Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1826.2 (2012): 407-414.
[20] Wertel, F., et al. "Myeloid and lymphoid dendritic cells in the peritoneal fluid of women with ovarian cancer." Adv Med Sci 51 (2006): 174-177.
[21] Boiocchi, Leonardo, et al. "BDCA-2 (CD303): a highly specific marker for normal and neoplastic plasmacytoid dendritic cells." Blood, The Journal of the American Society of Hematology 122.2 (2013): 296-297.
[22] Jaye, David L., et al. "Expression of the plasmacytoid dendritic cell marker BDCA-2 supports a spectrum of maturation among CD4+ CD56+ hematodermic neoplasms." Modern pathology 19.12 (2006): 1555-1562.




Comments
Leave a Comment