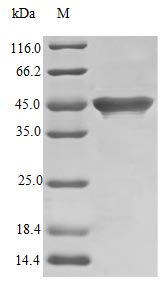
Recombinant Streptococcus sp Streptokinase G protein (skg) is produced in E. coli and contains the full-length mature protein spanning amino acids 27-440. The product comes without tags, which means no extra sequences interfere with its natural function. SDS-PAGE analysis shows purity levels above 97%, while endotoxin levels stay below 1.0 EU/µg according to LAL method testing. The protein appears to be fully biologically active, with a specific activity of 8.0 × 10^4 IU/mg demonstrated through fibrin lysis on agarose plate assays.
Streptokinase represents an important protein in medical research, largely because of its role in breaking down fibrin. It works as a plasminogen activator, transforming plasminogen into plasmin - a process that's essential for dissolving blood clots. This enzymatic pathway has drawn considerable attention for potential therapeutic uses in thrombolytic therapy, which makes streptokinase quite valuable for cardiovascular research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Fibrinolytic Activity Studies and Mechanism Research
This recombinant streptokinase G protein is confirmed to be biologically active (specific activity 8.0×10⁴ IU/mg in fibrin lysis) and suitable for fibrinolysis studies. However, the moderate specific activity suggests this may be a less potent streptokinase variant compared to some clinical isolates (which can reach 10⁵-10⁶ IU/mg). Researchers should validate activity in their specific assay systems and compare with established streptokinase standards. The high purity (>97%) supports reliable kinetic studies, but dose-response relationships may require higher concentrations than more potent variants.
2. Comparative Enzyme Activity Analysis
The protein enables valid comparative studies, but the moderate specific activity should be considered when comparing to other streptokinase variants or thrombolytic agents. Comparative analyses should include standardized activity units rather than mass-based comparisons. The agarose plate lysis assay provides a functional reference, but researchers should validate activity in solution-based assays for more quantitative kinetic comparisons.
3. Antibody Development and Immunological Studies
This high-purity, full-length streptokinase G (27-440aa) serves as a good antigen for antibody development. However, antibodies should be validated for specificity against other streptokinase variants and bacterial contaminants. The confirmed bioactivity ensures antibodies will recognize functional epitopes, but comprehensive validation should include testing against streptokinases from different bacterial strains to ensure variant specificity.
4. Protein-Protein Interaction Studies
The biologically active streptokinase is suitable for plasminogen interaction studies, but the moderate specific activity may indicate suboptimal plasminogen activator complex formation efficiency. Binding assays should validate that interaction kinetics match those of higher-activity streptokinase variants. The tag-free design supports authentic interactions, but researchers should confirm complex formation functionality in functional assays.
5. In Vitro Clot Lysis Assays and Screening Applications
The protein can be used for clot lysis studies, but the moderate potency may require higher concentrations for effective thrombolysis in some assay formats. Screening applications should establish system-specific dose-response curves rather than relying solely on the reported specific activity. The consistent purity supports reproducibility, but researchers should benchmark against clinical streptokinase standards for translational relevance.
Final Recommendation & Action Plan
This recombinant streptokinase G protein has confirmed fibrinolytic activity but exhibits moderate specific activity compared to some clinical variants, requiring an appropriate experimental design. First, establish a dose-response curve in your specific assay system to determine effective working concentrations, which may be higher than for more potent thrombolytics. For mechanistic studies, the high purity and tag-free design support reliable protein-interaction work, but validate that plasminogen activation kinetics match expected streptokinase functionality. When developing antibodies, this full-length protein provides comprehensive epitope coverage but validates specificity across streptokinase variants if strain discrimination is needed. For screening applications, use this protein as a reference standard, but include clinical streptokinase controls for comparative potency assessment. The E. coli expression produces authentic bacterial protein, but ensures the removal of any residual E. coli proteins in sensitive immunological applications. Always include appropriate fibrinolytic controls and consider that the moderate activity may make this protein more suitable for mechanistic studies rather than high-throughput screening, where higher potency may be desirable.