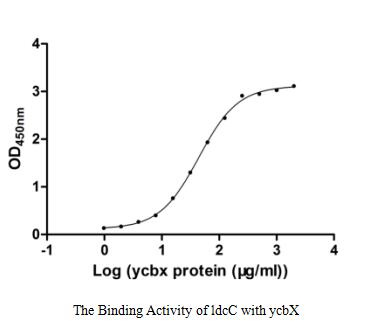
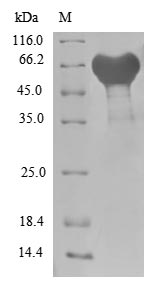
Recombinant Escherichia coli Uncharacterized protein YcbX (ycbX) is produced in an E. coli expression system and is available as a full-length protein from amino acids 1 to 369. The protein carries an N-terminal GST tag, which makes purification and detection more straightforward. SDS-PAGE analysis shows purity levels exceeding 85%, making this product suitable for research applications. Functional ELISA has confirmed biological activity, demonstrating binding to immobilized ldcC with an EC50 of 40.54-47.97 μg/ml.
The YcbX protein from Escherichia coli remains largely mysterious—its exact biological functions haven't been fully worked out yet. What we do know is that it can bind specific molecules, which suggests it may play a role in various cellular processes. As research continues, understanding how YcbX binds and what it does could shed light on its contribution to bacterial physiology and its potential connections to broader biological pathways.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Protein-Protein Interaction Studies with LdcC
This recombinant YcbX is confirmed to bind ldcC with moderate affinity (EC₅₀ 40.54-47.97 μg/ml) and is suitable for interaction studies. However, the moderate EC₅₀ may require high protein concentrations for detectable binding, and the N-terminal GST tag could sterically hinder interactions or cause non-specific binding. Researchers should validate binding kinetics with tag-free YcbX and include controls (e.g., GST alone) to ensure specificity. The E. coli expression system produces a homologous protein but may lack post-translational modifications present in native YcbX.
2. GST Pull-Down Assays for Identifying Binding Partners
The GST tag facilitates pull-down assays, but the 85% purity may increase non-specific binding from impurities. While useful for identifying novel interactors, researchers should include stringent controls (e.g., GST-only beads) and validate identified partners with native YcbX from E. coli lysates. The confirmed ldcC binding serves as a positive control, but the uncharacterized nature of YcbX requires caution in interpreting results.
3. Biochemical Characterization of an Uncharacterized E. coli Protein
This full-length YcbX can be used for initial characterization, but the binding activity to ldcC does not confirm enzymatic or broader functional roles. Researchers should perform additional assays (e.g., enzymatic screening, structural analysis) to determine YcbX's true function. The GST tag may alter biochemical properties, so tag-free protein is recommended for definitive characterization.
4. Development of Binding Assays and Screening Platforms
The protein is appropriate for developing binding assays (e.g., ELISA), but the moderate EC₅₀ may limit assay sensitivity for high-throughput screening. Researchers should optimize conditions (e.g., coating concentrations, detection methods) to improve dynamic range. The GST tag allows immobilization, but may affect ligand presentation; assay validation should include dose-response curves with known binders.
5. Antibody Development and Validation
This YcbX serves as an antigen for antibody generation, but the GST tag may induce tag-specific antibodies, reducing the yield of YcbX-specific antibodies. Antibodies should be affinity-purified against tag-free YcbX and validated for specificity in Western blot or immunofluorescence using E. coli cells expressing native YcbX. The 85% purity may require additional purification to minimize immune responses to contaminants.
Final Recommendation & Action Plan
This recombinant E. coli YcbX with an N-terminal GST tag is a functional tool for preliminary studies of YcbX-ldcC interactions and exploratory pull-down assays, but its moderate binding affinity, uncharacterized nature, and tag-related artifacts require careful validation. For immediate use, employ it in binding assays at concentrations based on the EC₅₀ (40-50 μg/ml), but include GST-tag controls to account for non-specific effects. For pull-downs, use glutathione beads for immobilization but validate novel interactions with native YcbX from E. coli lysates. For antibody development, immunize with this protein but screen hybrids against tag-free YcbX to ensure specificity. Given the protein's uncharacterized status, prioritize functional studies (e.g., enzymatic assays) to determine its biological role, and consider generating a tag-free version for critical applications. The 85% purity is acceptable for initial work but may need improvement for sensitive assays. Always use E. coli-specific controls to ensure biological relevance.