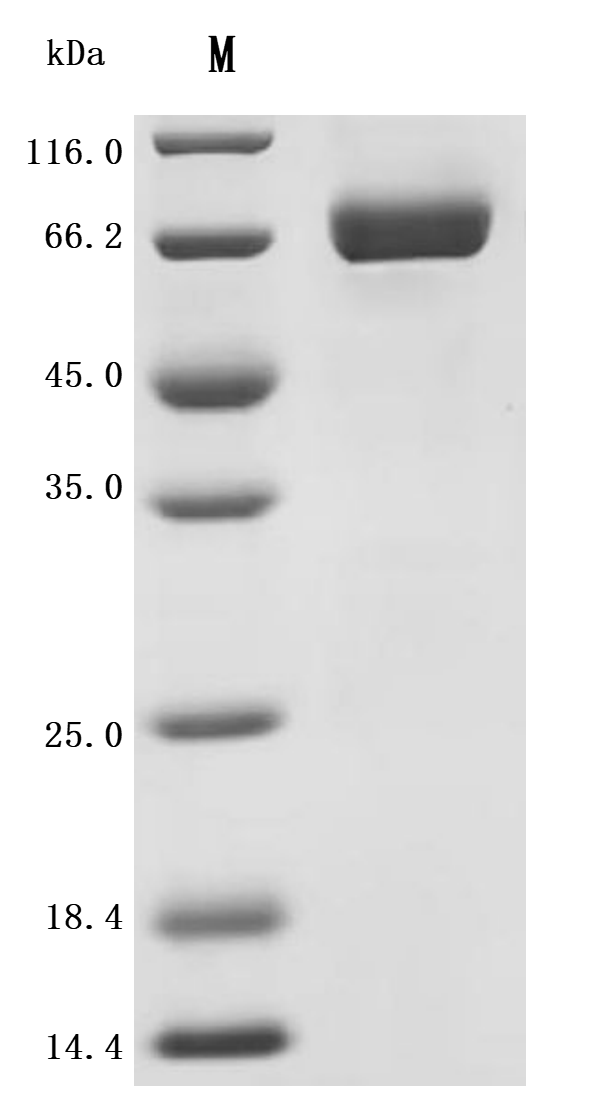
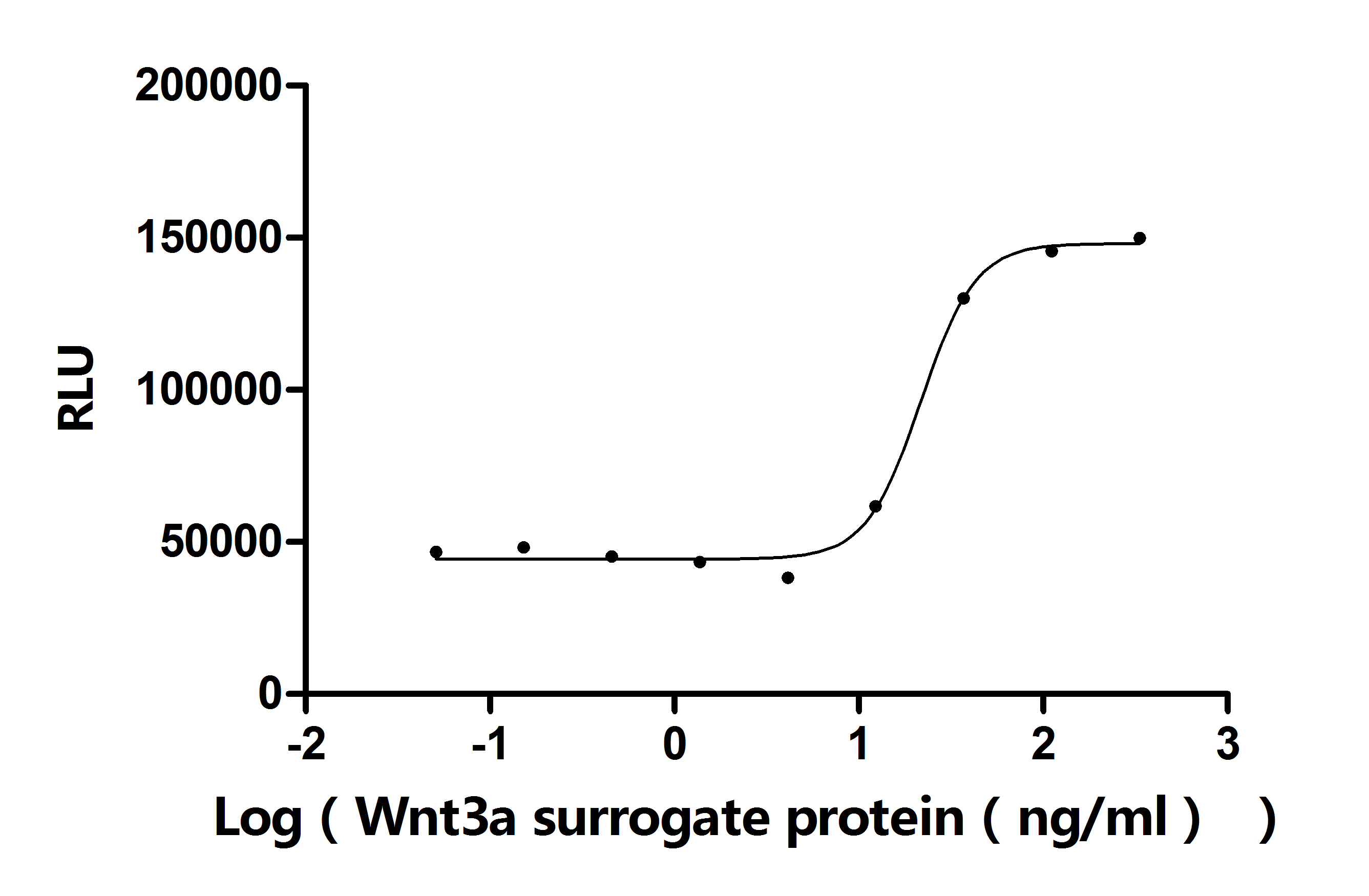
The recombinant human ITPRIPL1 protein is an active form expressed in a mammalian system to maintain native folding and post-translational modifications essential for functionality. It consists of amino acids 25 to 103, representing the extracellular domain of the human ITPRIPL1 protein, and is fused with a C-terminal 10xHis tag for ease of purification and detection. Supplied as a lyophilized powder, this recombinant ITPRIPL1 protein meets high-quality standards, with purity greater than 95% as confirmed by SDS-PAGE and endotoxin levels kept below 1.0 EU/µg, verified by the LAL assay. Functional ELISA results show that when immobilized at 2 μg/mL, ITPRIPL1 binds specifically to the anti-ITPRIPL1 recombinant antibody (CSB-RA756966MA1HU), with an EC50 ranging from 6.537 to 7.663 ng/mL. This makes it a reliable reagent for antibody validation, molecular interaction studies, and functional assays involving ITPRIPL1.
Human (ITPRIPL1 is a regulatory protein that has garnered interest due to its potential implications in various biological processes and pathologies, including cancer and immune responses. It is characterized as a low-expression gene often subjected to hypermethylation, particularly in breast cancer (BC), which may have prognostic significance in this condition. Specifically, ITPRIPL1 has been identified as an independent prognostic biomarker influencing the survival rates of BC patients, indicating its role in cancer biology and potentially tumor immune evasion mechanisms [1].
The function of ITPRIPL1 is intrinsically linked to its interaction with inositol 1,4,5-trisphosphate receptors (IP3Rs), which are crucial for calcium signaling within cells. These receptors facilitate the release of Ca²⁺ from the endoplasmic reticulum following activation by their ligand, inositol 1,4,5-trisphosphate (IP3). The interactions between ITPRIPL1 and IP3Rs are pivotal, as they can regulate various cellular events, including apoptosis, secretion, and gene expression [2][3][4]. For instance, it has been shown that modulators of calcium signaling involving IP3Rs may influence immune responses, wherein ITPRIPL1 could play a role that potentially impacts T cell activation and subsequent immune tolerance in certain physiological contexts [1].
Furthermore, ITPRIPL1's relationship with calcium signaling highlights its potential role in important physiological processes, including those related to secretion systems in endocrine and exocrine tissues. For example, ITPRIPL1 may influence the function of proteins associated with the exocytosis of hormones and digestive enzymes through its interactions with IP3Rs, further implicating it in metabolic regulation and signaling pathways pertinent to these processes [5].
Notably, research also hints at the involvement of ITPRIPL1 in male reproductive biology, particularly due to its enrichment in spermatids, suggesting a function in the immune privilege of reproductive organs [1]. This indicates that ITPRIPL1 might have specialized roles depending on tissue types and physiological states, aligning with its broader implications in health and disease.
References:
[1] X. Zhong and G. Zhong. Prognostic biomarker identification and tumor classification in breast cancer patients by methylation and transcriptome analysis. Febs Open Bio, vol. 11, no. 8, p. 2139-2151, 2021. https://doi.org/10.1002/2211-5463.13211
[2] S. Deng, Y. Wang, J. Brosseau, Y. Wang, & J. Xu. The itpripl1- cd3ε axis: a novel immune checkpoint controlling t cells activation., 2022. https://doi.org/10.1101/2022.02.25.481189
[3] H. Ando, A. Mizutani, H. Kiefer, D. Tsuzurugi, T. Michikawa, & K. Mikoshiba. Irbit suppresses ip3 receptor activity by competing with ip3 for the common binding site on the ip3 receptor. Molecular Cell, vol. 22, no. 6, p. 795-806, 2006. https://doi.org/10.1016/j.molcel.2006.05.017
[4] L. Xia, D. Zhang, C. Wang, F. Wei, & Y. Hu. Pc‐plc is involved in osteoclastogenesis induced by tnf‐α through upregulating ip3r1 expression. Febs Letters, vol. 586, no. 19, p. 3341-3348, 2012. https://doi.org/10.1016/j.febslet.2012.07.015
[5] D. Rossum, R. Patterson, et al. Danger, a novel regulatory protein of inositol 1,4,5-trisphosphate-receptor activity. Journal of Biological Chemistry, vol. 281, no. 48, p. 37111-37116, 2006. https://doi.org/10.1074/jbc.m608760200b