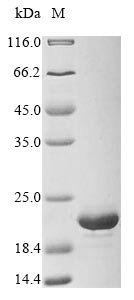
Recombinant Staphylococcus epidermidis Endoribonuclease MazF is produced in E. coli and includes an N-terminal 6xHis-tag that makes purification and detection more straightforward. The protein appears to be expressed as a complete construct covering amino acids 1-120. SDS-PAGE analysis confirms purity levels exceeding 90%, which suggests this product may be well-suited for research applications requiring high-quality recombinant proteins.
Endoribonuclease MazF is recognized for its RNA cleavage activity—it seems to recognize and cut RNA at particular sequences. Bacterial stress responses likely involve this enzyme, and researchers have been examining its role in pathways that regulate cell growth. MazF's capacity to target RNA makes it potentially valuable for studies focused on gene expression control and how cells respond to stress.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Staphylococcus epidermidis MazF is a bacterial toxin protein that functions as a sequence-specific endoribonuclease in toxin-antitoxin systems. The E. coli expression system is homologous to this bacterial protein, significantly increasing the probability of correct folding. MazF requires precise tertiary structure formation for its RNA cleavage activity but does not typically require complex post-translational modifications. The N-terminal 6xHis tag is small (∼2 kDa) and unlikely to sterically interfere with the protein's active site or functional domains. Therefore, this recombinant MazF has a high probability of being correctly folded and functionally active.
1. Protein-Protein Interaction Studies Using His-Tag Affinity Purification
The native bacterial expression system and small tag support authentic protein-protein interactions with minimal interference. This application is conditionally suitable but requires validation of protein folding. If properly folded, this recombinant MazF is highly suitable for studying interactions with potential regulatory partners, including its cognate antitoxin MazE. Pull-down assays can reliably identify physiological interactors involved in toxin-antitoxin regulation. The >90% purity minimizes non-specific binding. If misfolded, any interaction data is invalid.
2. Biochemical Characterization and Enzyme Kinetics Analysis
This is a priority application for validating functional activity. The protein can be used in ribonuclease assays with specific RNA substrates to determine cleavage specificity, kinetic parameters (Km, Vmax), and optimal reaction conditions. Thermal stability and pH optimum studies can characterize the enzyme's biochemical properties. Perform a pre-experiment first.
3. Antibody Development and Immunological Applications
This recombinant MazF serves as an excellent immunogen for generating specific antibodies against S. epidermidis MazF. The full-length sequence ensures comprehensive epitope coverage. The high purity (>90%) minimizes antibodies against contaminants. These antibodies will be valuable for detecting MazF expression in bacterial cultures.
4. Comparative Structure-Function Studies
This protein is ideal for comparative studies with MazF homologs from other bacterial species after its folding and activity have been validated. The properly folded, active enzyme enables meaningful functional comparisons across bacterial species and mutagenesis studies. Functional comparisons of cleavage specificity, inhibitor sensitivity, and kinetic parameters can reveal evolutionary adaptations in toxin-antitoxin systems. Site-directed mutagenesis studies can identify critical residues for catalytic activity.
Final Recommendation & Action Plan
This recombinant MazF expressed in its homologous E. coli system with a minimal His-tag is highly likely to be properly folded and functionally active, making it suitable for all proposed applications. The recommended approach is to begin with Application 2 (Biochemical Characterization) to confirm ribonuclease activity with specific RNA substrates and establish kinetic parameters. Once activity is validated, proceed confidently with Applications 1, 3, and 4 for interaction studies, antibody development, and comparative analyses. For all applications, the high purity and native folding make this reagent reliable for both structural and functional studies of bacterial toxin-antitoxin systems.