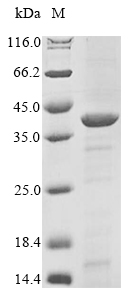
Recombinant Acinetobacter baumannii UDP-3-O-acyl-N-acetylglucosamine deacetylase (lpxC) is produced in E. coli with an N-terminal 6xHis tag to simplify purification. The protein is expressed as a full-length form, spanning amino acids 1 to 300. SDS-PAGE analysis confirms it reaches a purity level of greater than 85%, which appears to provide a high-quality reagent for experimental applications.
LpxC protein plays a crucial role in the lipid A biosynthesis pathway—a component of the bacterial outer membrane. As a deacetylase, it catalyzes the removal of an acetyl group from UDP-3-O-acyl-N-acetylglucosamine. This represents a key step in lipid A biosynthesis. Understanding this enzyme's activity may be essential for research into bacterial cell wall synthesis and potential antibiotic target development.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Acinetobacter baumannii LpxC is a zinc-dependent metalloenzyme that requires precise folding and metal cofactor binding for its deacetylase activity in lipid A biosynthesis. The E. coli expression system is compatible with this bacterial protein, increasing the likelihood of correct folding. However, the N-terminal 6xHis tag may potentially interfere with the enzyme's active site or metal binding pocket. While the full-length protein (1-300aa) contains all functional domains, the probability of correct folding with full enzymatic activity is moderate but requires experimental validation due to potential tag interference.
1. Biochemical Characterization and Enzyme Kinetics Studies
This application is conditionally suitable but requires activity validation first. Enzymatic function requires precise active site structure and metal cofactor binding that may be compromised by the tag. If the protein is correctly folded and retains zinc-binding capability, it can be used for kinetic studies (Km, Vmax determination). However, the His-tag may affect metal cofactor binding or substrate access, potentially altering kinetic parameters. Initial activity assays with appropriate controls are essential.
2. Inhibitor Screening and Drug Discovery Research
This application carries a significant risk without prior activity confirmation. A misfolded or inactive LpxC will yield false negatives in inhibitor screens. If activity is validated, the protein becomes highly valuable for screening A. baumannii-specific LpxC inhibitors, which are clinically relevant for this multidrug-resistant pathogen.
3. Antibody Development and Immunological Studies
This recombinant LpxC serves as an excellent immunogen for generating antibodies against A. baumannii LpxC. The full-length sequence ensures comprehensive coverage of the epitope. The His-tag facilitates purification and screening. These antibodies will be valuable for detecting LpxC in bacterial samples.
4. Protein-Protein Interaction Studies
Protein-protein interactions require native conformation that may be affected by folding status and tag presence. If correctly folded, LpxC could identify physiological interaction partners in lipid A biosynthesis. However, misfolding may cause non-specific binding or failure to interact. The His-tag may sterically hinder some interactions. Results require validation with complementary methods.
Final Recommendation & Action Plan
This recombinant LpxC has potential for multiple applications but requires rigorous validation of enzymatic activity before reliable use in functional studies. The recommended approach is to first validate zinc-dependent deacetylase activity using established spectrophotometric or HPLC-based assays with authentic substrate. If activity is confirmed, proceed with Applications 1 and 2 (kinetic studies and inhibitor screening). Application 3 (antibody development) can proceed immediately regardless of functional status. Application 4 (interaction studies) should include appropriate controls and validation steps. If activity is low, focus on structural and immunological applications only. For reliable drug discovery applications, consider tag removal or use of tag-free LpxC to ensure native conformation. This systematic validation ensures appropriate use based on functional capability.