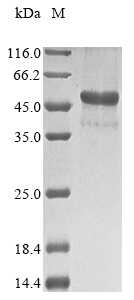
The synthesis of recombinant Amylomyces rouxii Chitin deacetylase involves isolating the target gene, which encodes the full length of the mature chitin deacetylase. This gene is linked with a10xHis-tag gene at the N-terminus and then cloned into an appropriate expression vector. The vector is transfected into E. coli cells, which are cultured for the expression of the recombinant protein. The recombinant protein is isolated and purified from the cell lysate using affinity chromatography. Its purity exceeds 85% as determined by SDS-PAGE.
Chitin deacetylase is an enzyme that catalyzes the deacetylation of chitin, a structural biopolymer found in various marine life forms, fungal cell walls, spores, insect cuticles, and peritrophic matrices [1]. The deacetylation process carried out by chitin deacetylases, such as the one found in Amylomyces rouxii, involves the hydrolysis of N-acetamido groups of N-acetyl-D-glucosamine residues in chitin [2]. Chitin deacetylases, including those found in Amylomyces rouxii, are essential for cell wall morphogenesis and remodeling in fungi and bacteria [3]. They are also used by pathogenic microorganisms to evade host defense [3].
References:
[1] Z. Yong, R. Park, & R. Müzzarelli, Chitin deacetylases: properties and applications, Marine Drugs, vol. 8, no. 1, p. 24-46, 2010. https://doi.org/10.3390/md8010024
[2] C. Mishra, C. Semino, K. McCreath, H. Vega, B. Jones, C. Spechtet al., Cloning and expression of two chitin deacetylase genes ofsaccharomyces cerevisiae, Yeast, vol. 13, no. 4, p. 327-336, 1997. https://doi.org/10.1002/(sici)1097-0061(19970330)13:43.0.co;2-t
[3] H. Aragunde, X. Biarnés, & A. Planas, Substrate recognition and specificity of chitin deacetylases and related family 4 carbohydrate esterases, International Journal of Molecular Sciences, vol. 19, no. 2, p. 412, 2018. https://doi.org/10.3390/ijms19020412