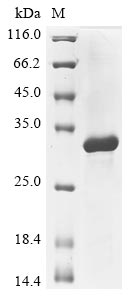
Recombinant Anemonia sulcata GFP-like non-fluorescent chromoprotein FP595 is produced in E. coli, spanning the complete mature protein sequence from amino acids 1 to 62. The protein carries an N-terminal GST tag and shows purity levels exceeding 85% when analyzed by SDS-PAGE. This product is designed exclusively for research purposes and lacks fluorescence properties while maintaining low endotoxin concentrations suitable for experimental work.
FP595 represents a GFP-like chromoprotein sourced from the Mediterranean snakelocks sea anemone, Anemonia sulcata. What sets this protein apart from its fluorescent relatives is its complete lack of fluorescence, which may make it particularly valuable across different research contexts. The distinctive chromophore characteristics appear to be well-suited for studies examining protein interactions, stability testing, and serving as a control in experiments requiring non-fluorescent markers.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant FP595 chromoprotein is unlikely to be correctly folded or bioactive without experimental validation. GFP-like proteins require precise folding to form a functional chromophore, which involves cyclization and oxidation reactions that may not occur properly in the E. coli expression system. The large N-terminal GST tag (∼26 kDa) is substantially larger than the FP595 domain (∼7 kDa, 62 aa) and may sterically hinder correct folding, particularly if the chromophore formation requires accessibility of the N-terminal region.
1. Protein Expression and Purification Optimization Studies
This GST-tagged chromoprotein can be used as a model for optimizing expression and purification protocols in E. coli, as this application primarily relies on the GST tag's affinity properties rather than protein functionality. However, if the protein is misfolded, it may not serve as an accurate representative for other fluorescent/chromoproteins, as folding conditions could differ. For reliable protocol development, include validation of chromophore formation or use a well-characterized control protein.
2. Antibody Development and Validation
This recombinant protein can generate antibodies, but if misfolded, antibodies may recognize non-native epitopes or the GST tag rather than the native chromoprotein. For antibodies specific to natural FP595, validate using correctly folded protein or endogenous material from Anemonia sulcata. The high purity reduces contamination risks but does not ensure antigen quality.
Final Recommendation & Action Plan
To ensure reliable results, first validate the folding and chromophore formation of the recombinant FP595 using absorption spectroscopy (e.g., check for the characteristic ∼595 nm peak) and circular dichroism to confirm secondary structure. If the GST tag is suspected to interfere, remove it via proteolytic cleavage (if a cleavage site is present) and re-purify the tag-free protein for functional studies. For applications like antibody development or interaction assays, always include controls such as GST-only proteins or well-folded chromoprotein standards. If folding cannot be verified, limit use to tag-based applications (e.g., purification optimization) and avoid functional or comparative studies.