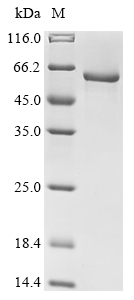
Recombinant Arabidopsis thaliana At1g09870 is a full-length mature protein expressed in E. coli, covering the amino acid region 20-487. It includes an N-terminal 10xHis-tag for streamlined purification and detection. SDS-PAGE analysis indicates the protein demonstrates a purity greater than 90%, which appears to ensure reliable performance for research applications. This product is designed for research use only and is not intended for diagnostic or therapeutic purposes.
At1g09870 is a protein from Arabidopsis thaliana, commonly used as a model organism in plant biology. This protein seems to be involved in various cellular processes and pathways that may be essential for plant development and stress responses. Studying At1g09870 could provide insights into plant biology and contribute to a broader understanding of fundamental biological processes in eukaryotic organisms.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The E. coli expression system is unlikely to produce a correctly folded version of this Arabidopsis thaliana protein (At1g09870) with functional activity. Plant proteins often require specific eukaryotic post-translational modifications, chaperones, and cellular environments that bacterial systems cannot provide. While the protein may be soluble, the probability of correct tertiary structure formation and biological activity is low. The "Full Length of Mature Protein" designation suggests the signal peptide may have been removed, but proper folding remains uncertain.
1. Antibody Development and Validation
This recombinant At1g09870 serves as an excellent immunogen for generating antibodies against linear epitopes. The full-length sequence ensures comprehensive epitope coverage. However, antibodies may not efficiently recognize conformational epitopes on the native protein in plant tissues.
2. Biochemical Characterization
This is the essential first step to assess the protein's physical properties. Techniques like circular dichroism can analyze secondary structure, while thermal shift assays can evaluate stability. These studies provide critical quality control data but characterize a bacterially expressed variant, not the native plant protein.
Final Recommendation & Action Plan
This recombinant plant protein expressed in E. coli is primarily suitable for antibody development and biochemical characterization but should not be used for interaction studies without validation. The recommended approach is to prioritize Application 2 (Biochemical Characterization) to assess the protein's folding state and stability. Application 1 (Antibody Development) can proceed immediately for generating linear epitope antibodies. Protein-protein interactions require precise three-dimensional conformation that E. coli likely cannot provide for this plant protein. Protein complex formation requires properly folded proteins with correct interaction interfaces. A misfolded protein will not form physiological complexes and may instead create artifactual associations. For functional studies, alternative approaches using plant-based expression systems or endogenous protein from Arabidopsis tissues are necessary.