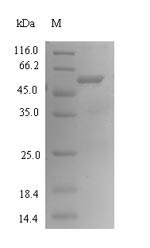
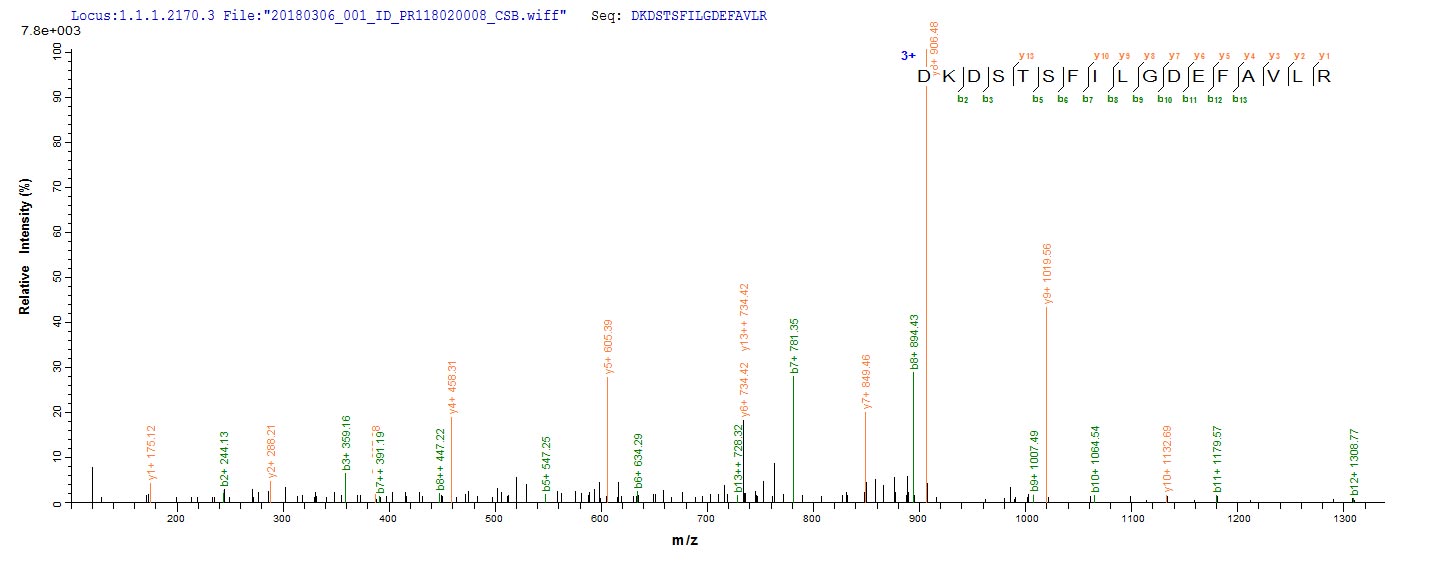
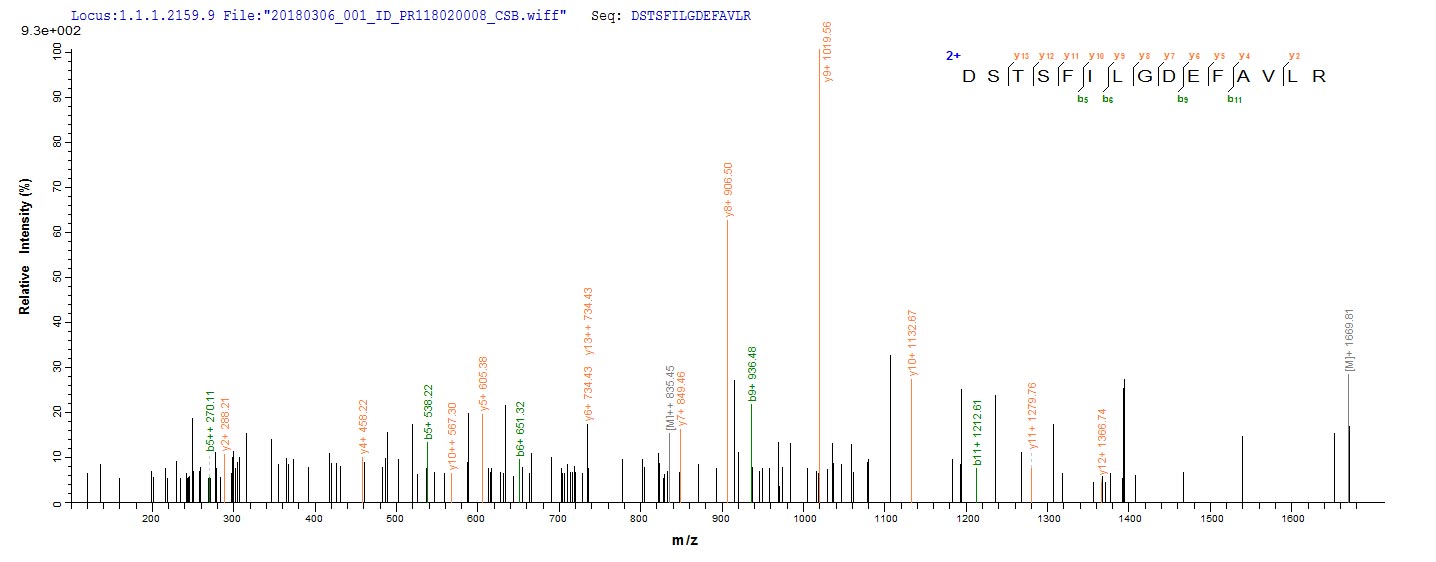
Recombinant Bacteroides fragilis Fragilysin (btfP) is expressed in E. coli and includes an N-terminal 6xHis-SUMO tag that helps with purification and appears to improve stability. The protein covers the complete mature form, from amino acids 26 to 405. SDS-PAGE analysis shows it reaches purity levels above 90%. This product is intended for research use only and seems to offer consistent quality for experimental work.
Fragilysin is a metalloprotease that Bacteroides fragilis produces—a bacterium that's quite common in the human gut. This protein breaks down extracellular matrix components, which likely helps the bacteria establish themselves and may contribute to disease processes. The enzymatic activity of fragilysin has become a key focus for researchers studying microbial interactions and its potential role in gastrointestinal diseases, making it an important target for scientific investigation.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Bacteroides fragilis Fragilysin is expressed in E. coli. Fragilysin is a zinc-metalloprotease toxin that requires precise folding and likely disulfide bond formation for its structural stability and activity. While E. coli can express bacterial proteins, fragilysin's functional conformation depends on correct propeptide processing and zinc coordination, which may not occur properly in E. coli without specific optimization. The N-terminal 6xHis-SUMO tag may improve solubility, but doesn't guarantee native folding. Since activity is unverified, the protein may be misfolded or inactive. The expression of the mature protein region (26-405aa) is favorable, but without functional validation, correct folding cannot be assumed.
1. Protein-Protein Interaction Studies Using Pull-Down Assays
The N-terminal 6xHis-SUMO tag facilitates purification and immobilization for pull-down experiments. However, if fragilysin is misfolded, it may not interact authentically with biological partners, potentially identifying non-physiological interactions. The SUMO tag's stability benefits are offset by the risk of misfolding. This application should only be pursued after confirming the protein's native conformation, as results from an incorrectly folded toxin would be misleading for understanding its molecular mechanisms.
2. Antibody Development and Immunoassay Applications
This application is generally suitable. The recombinant fragilysin can serve as an effective immunogen for antibody production, as antibodies often recognize linear epitopes that are less dependent on native folding. The high purity (>90%) and full-length mature protein sequence make it appropriate for generating antibodies useful in techniques like Western blotting or ELISA. However, antibodies against a potentially misfolded protein might not recognize the native, functionally active fragilysin in bacterial cultures, so validation against the native protein is recommended.
3. Biochemical Characterization and Stability Studies
This application is highly appropriate and should be prioritized. Techniques like size exclusion chromatography, dynamic light scattering can directly assess the protein's oligomeric state, folding quality, and stability. These studies are essential to determine whether the recombinant protein is properly folded before proceeding to functional assays. The high purity supports reliable characterization. Even if the protein is misfolded, these analyses provide valuable data about the recombinant product's properties.
4. Tag Removal and Functional Domain Analysis
This application is theoretically sound but depends on correct folding after tag cleavage. SUMO protease cleavage can yield untagged fragilysin, but if the protein is misfolded before cleavage, removal of the SUMO tag won't restore function. Comparing tagged versus untagged versions is valid for assessing tag interference, but this comparison is only meaningful if the core protein is properly folded. Functional assays must be performed post-cleavage to confirm activity. The approach is correct, but its success hinges on initial folding quality.
Final Recommendation & Action Plan
Given the uncertainty in folding and bioactivity, the recommended approach is to first conduct comprehensive biochemical characterization (Application #3) to assess the protein's conformational state, followed by tag cleavage and functional validation (Application #4). Priority should be given to testing proteolytic activity using known substrates (e.g., casein or specific peptide substrates) to confirm bioactivity. If the protein demonstrates expected enzymatic function, it can be cautiously used for interaction studies (Application #1), with verification of findings through complementary methods. For antibody development (Application #2), the protein can be used immediately, with the understanding that resulting antibodies may require validation against native fragilysin. Always include appropriate controls and consider the potential need for refolding optimization if initial characterization reveals misfolding.