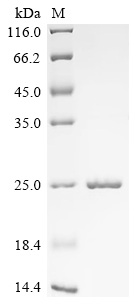
Recombinant Bovine Odorant-binding protein is produced in E. coli, covering the full-length amino acid sequence from 1 to 159. This product is tag-free and achieves a purity level of over 85%, as verified by SDS-PAGE analysis. It's designed for research use with a focus on accuracy and reliability, ensuring minimal endotoxin interference to suit various scientific applications.
Odorant-binding proteins appear to be integral to the olfactory system, carrying odor molecules to receptors. In bovines, these proteins may play a crucial role in detecting and binding odorants, which is essential for understanding olfactory signaling pathways and the mechanisms of scent detection. This recombinant protein serves as a valuable tool for research into sensory biology and protein-ligand interactions.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Bovine odorant-binding protein (OBP) is a small soluble protein that typically requires proper folding to form a hydrophobic binding pocket for odorants. The E. coli expression system can often produce soluble versions of such proteins, but the presence of an N-terminal S-tag (a 15-amino acid tag) may slightly affect the protein's N-terminal structure or stability. However, since OBP is not a complex multidomain protein and the tag is small, the protein has a moderate to high likelihood of being correctly folded. Its activity (odorant binding) should be experimentally verified but is feasible.
1. Protein-Protein Interaction Studies
Functional binding studies depend on native conformation. The small S-tag may not severely hinder interactions, but activity must be verified before reliable use. If the protein is correctly folded, it could be used to study interactions with odorants or other proteins (e.g., receptors) via SPR or BLI. However, the S-tag might sterically interfere with some interactions, and the protein's binding activity must be confirmed first. Negative results could arise from misfolding or tag interference.
2. Biochemical Characterization and Stability Studies
This is a priority application to assess the protein's folding and stability. Techniques like DSF, DLS, and circular dichroism can determine thermal stability, oligomeric state, and secondary structure. The S-tag is small and unlikely to significantly alter biophysical properties, making this application highly reliable for characterizing the protein's intrinsic properties. These studies are essential for quality control and can be performed regardless of functional activity, providing data on folding and stability.
3. Antibody Development and Validation
This recombinant OBP is highly suitable as an immunogen for antibody production. The full-length sequence ensures broad epitope coverage, and the S-tag may even provide an additional epitope for screening. Antibodies can be validated using Western blot, ELISA, or immunohistochemistry, with the protein serving as a positive control.
4. Comparative Structural and Functional Analysis
This protein can be used for comparative studies with OBPs from other species, focusing on structural features (e.g., via circular dichroism) or sequence conservation. However, functional comparisons (e.g., odorant binding affinity) require prior validation of this protein's activity. The S-tag may slightly affect structural comparisons but not significantly.
Final Recommendation & Action Plan
This recombinant OBP is likely to be correctly folded due to its simple structure and small tag, but functional activity must be confirmed before use in binding or comparative functional studies. The immediate priority is Application 2 (Biochemical Characterization) to verify folding and stability via DSF, DLS, or circular dichroism. If the protein shows native-like structure, proceed to validate odorant-binding activity using a known ligand (e.g., fluorescent odorant binding assay). Once activity is confirmed, Applications 1 and 4 (interaction studies and functional comparisons) can be pursued. Application 3 (Antibody Development) can proceed immediately regardless of functional status. If activity is low, focus on structural and immunological applications only. This stepwise approach ensures reliable outcomes.