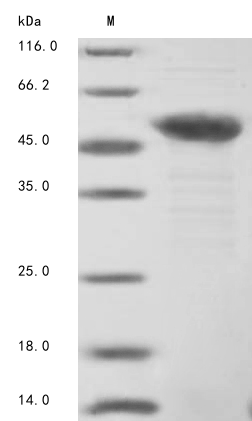
Recombinant Bovine Troponin T, fast skeletal muscle (Tnnt3), comes from E. coli production and appears as a full-length mature protein covering amino acids 2 to 271. The protein carries a 10xHis tag at the N-terminus and a Myc tag at the C-terminus for purification and detection purposes. This recombinant protein achieves a purity level exceeding 85%, as confirmed through SDS-PAGE analysis, which makes it suitable for various research applications.
Troponin T forms part of the troponin complex and plays a crucial role in muscle contraction regulation through its binding to tropomyosin. Fast skeletal muscle fibers contain this protein, and it appears vital for the calcium-mediated signaling pathway that controls muscle contraction and relaxation. Research into muscle physiology and related disorders likely depends on understanding how Troponin T interacts and functions.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, recombinant bovine Troponin T (Tnnt3) is produced in an E. coli expression system as the full-length mature protein (2-271aa) with both N-terminal 10xHis and C-terminal Myc tags. Troponin T is a complex structural muscle protein that requires specific folding and proper interaction domains for its biological function in calcium-dependent muscle contraction regulation. E. coli expression systems often struggle with correct folding of eukaryotic structural proteins that require specific post-translational modifications or chaperone assistance. The dual tagging system, particularly the large tags at both termini, may significantly interfere with the protein's native conformation and functional domains. No validation data (e.g., complex formation assays, circular dichroism) are provided. Therefore, the protein's folding and bioactivity cannot be confirmed and are likely compromised.
This E. coli-expressed dual-tagged Tnnt3 is unsuitable for functional studies involving troponin complex formation or muscle contraction mechanisms due to the high probability of improper folding and tag interference. Antibody development can proceed with the understanding that resulting antibodies may have limited utility for conformational epitope recognition. The high purity and full-length sequence provide comprehensive epitope coverage for generating Tnnt3-specific antibodies. However, antibodies produced against misfolded protein may not optimally recognize native troponin T in physiological contexts, particularly for conformation-dependent epitopes. The tags provide additional epitopes that could be advantageous for detection, but may also lead to tag-specific antibodies. For reliable Tnnt3 research, alternative expression systems such as eukaryotic hosts (e.g., insect or mammalian cells) that better support proper folding of muscle proteins should be considered, or commercially validated native troponin complexes should be used as benchmarks.