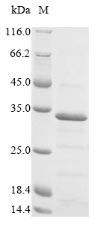
Recombinant Chicken anemia virus Apoptin (VP3) is expressed in E. coli, covering the full-length expression region from 1-121 amino acids. The protein includes an N-terminal 6xHis-SUMO tag, which should make purification relatively straightforward. Purity appears to exceed 90% based on SDS-PAGE analysis, suggesting it may be suitable for most research applications. Endotoxin levels have been kept low to avoid interference with sensitive experimental conditions.
Apoptin, also known as VP3, comes from the Chicken anemia virus and seems to have an interesting property - it can trigger cell death in transformed and cancer cells while leaving normal cells largely unaffected. This selective behavior makes it potentially useful for investigating cellular pathways involved in programmed cell death, cell cycle control, and cancer biology. The protein might offer clues about new therapeutic strategies, though much remains to be understood about its mechanisms.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Chicken anemia virus Apoptin (VP3) is a viral protein that requires specific phosphorylation and nuclear localization for its selective apoptosis-inducing activity in transformed cells. The E. coli expression system cannot perform the necessary eukaryotic post-translational modifications (particularly phosphorylation at Thr108) that are critical for Apoptin's functional conformation and cancer-selective toxicity. The large N-terminal SUMO tag (∼15 kDa) may sterically interfere with the protein's functional domains and nuclear localization signals. While the protein may be soluble, it is highly unlikely to achieve the correct folding and modification needed for functional activity.
1. Antibody Development and Characterization
This recombinant Apoptin serves as an excellent immunogen for generating antibodies against linear epitopes of CAV VP3. The full-length sequence ensures comprehensive coverage of the epitope. The SUMO tag facilitates purification and immunization procedures. These antibodies will be valuable for detecting Apoptin in Western blot applications.
2. Biochemical Characterization and Biophysical Analysis
This is the essential first step to assess the protein's physical properties. Techniques like size-exclusion chromatography can determine oligomeric state, while circular dichroism can analyze secondary structure content and thermal stability. SUMO protease cleavage allows comparison of tagged versus untagged protein properties. These analyses provide crucial quality control data about the protein itself, not the native Apoptin.
Final Recommendation & Action Plan
This recombinant Apoptin is primarily suitable for antibody development and biochemical characterization but fundamentally unsuitable for functional studies due to E. coli's inability to produce the phosphorylated, correctly folded protein required for Apoptin's unique cancer-selective activity. The immediate priority is Application 2 (Biochemical Characterization) to assess the protein's physical properties. Application 1 (Antibody Development) can proceed immediately for generating linear epitope antibodies. Apoptin's functional interactions require precise tertiary structure and phosphorylation that this bacterially expressed variant cannot provide. Apoptin's cellular localization and trafficking studies require the native, properly modified protein to produce biologically relevant results. For functional Apoptin studies, the only valid approach is to use the protein expressed in eukaryotic systems (e.g., mammalian cells) where it can undergo proper phosphorylation and folding.