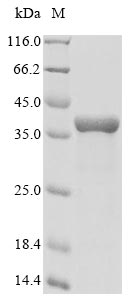
Recombinant Drosophila melanogaster Pro-resilin is a research-grade protein expressed in E. coli, featuring a partial sequence from amino acids 342 to 620. This protein contains N-terminal 10xHis and C-terminal Myc tags for streamlined purification and detection. SDS-PAGE analysis confirms the protein achieves over 90% purity, which appears sufficient for most experimental applications where high purity is essential.
Pro-resilin represents a structural protein found in the cuticle of Drosophila melanogaster. What makes it particularly interesting is its exceptional elastic properties. The protein seems to play a crucial role in providing elasticity and resilience to insect tissues—characteristics that are vital for movement and mechanical stability. These unique features have caught the attention of biomaterials researchers, who are studying its mechanical properties for potential applications in new material development.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the folding state and bioactivity of this recombinant resilin protein fragment are unknown and must be considered highly uncertain. Resilin is an elastic protein that requires specific post-translational modifications (e.g., di- and trityrosine cross-linking) for its native function, which are unlikely to occur in the reducing cytoplasm of E. coli. The expression of a partial fragment (342-620aa) with large tags on both termini (N-terminal 10xHis and C-terminal Myc) further complicates correct folding, as the tags may sterically hinder the protein's natural conformation. Therefore, it is highly improbable that this protein is correctly folded or bioactive for its native elastic properties. Applications relying on specific biological interactions or the protein's native structure are speculative without validation.
1. Biochemical Characterization of Resilin Protein Properties
This purified recombinant protein is suitable for basic biochemical and biophysical characterization. Techniques like amino acid analysis, circular dichroism, and stability studies can be applied to study this specific fragment. This application is valid as it focuses on the intrinsic properties of the polypeptide, independent of native bioactivity. However, the data will characterize the recombinant fragment rather than the native resilin network, so comparisons with to full-length protein should be made cautiously.
2. Development of Anti-Resilin Research Antibodies
The recombinant resilin protein can be used as an immunogen to generate antibodies. The high purity minimizes cross-reactivity issues. However, the antibodies generated will recognize linear epitopes of this non-cross-linked, bacterially expressed fragment. Their ability to recognize the naturally cross-linked, mature resilin in Drosophila tissues is very low and requires rigorous validation. These antibodies are best suited for detecting denatured resilin in Western blots.
3. Tag-Based ELISA Development for Resilin Detection
The dual tags enable sandwich ELISA development. However, the utility is limited to detecting the recombinant resilin protein itself. The assay will not accurately quantify native resilin in biological samples, as the epitopes may be masked or altered in the cross-linked native form. Such an ELISA is only for research on the recombinant standard, not for endogenous resilin detection.
Final Recommendation & Action Plan
The immediate recommendation is to restrict the use of this protein to applications that do not depend on native folding or bioactivity, such as biochemical characterization (Application 1) and antibody production for linear epitope detection (Application 3). Prior to any functional studies, the protein's folding should be assessed using techniques like circular dichroism or size-exclusion chromatography, and if possible, compared to native resilin.