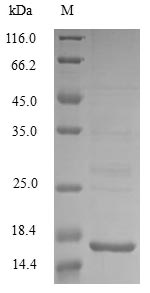
Recombinant Escherichia coli Acidic protein msyB is produced through a baculovirus expression system, which includes the complete protein sequence from amino acids 1 to 124. A C-terminal 6xHis-tag has been added to make purification and detection more straightforward. SDS-PAGE analysis suggests the product achieves purity levels above 85%, making it well-suited for research applications that demand high-quality recombinant proteins.
The msyB protein from Escherichia coli appears to participate in several cellular processes. This makes it a useful tool for investigating bacterial physiology and molecular mechanisms. Research indicates it may be particularly important for understanding how protein interactions work and how cells respond in prokaryotic systems. Scientists are also exploring its role in bacterial regulatory pathways and possible biotechnology uses.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
While the baculovirus expression system generally promotes proper eukaryotic protein folding, its ability to correctly fold a bacterial protein like E. coli msyB is uncertain. The eukaryotic folding environment may lack specific chaperones or conditions optimal for this prokaryotic protein's native conformation. Therefore, the probability of correct folding is moderate but not guaranteed. Consequently, the protein's functional activity remains unknown and cannot be assumed without experimental validation.
1. Protein-Protein Interaction Studies Using His-Tag Pull-Down Assays
The utility of this recombinant msyB for interaction studies is critically dependent on correct folding. If natively folded, it could identify physiological binding partners. However, if misfolded, it may expose non-native hydrophobic surfaces, leading to non-specific (false-positive) binding, or mask the genuine interaction interface, causing false-negative results. Data would be uninterpretable without independent confirmation of its native structure.
2. Antibody Development and Validation
This recombinant msyB serves as an excellent immunogen for generating specific antibodies. The full-length sequence ensures broad epitope coverage. Even if misfolded, it will generate antibodies against linear epitopes useful for techniques like Western blotting. The dual tags simplify purification and screening. If correctly folded, it may also elicit antibodies recognizing conformational epitopes present on the native protein in E. coli.
3. Biochemical Characterization and Stability Studies
This application is the essential first step to determine the protein's physical state. Techniques like size-exclusion chromatography (SEC) can assess oligomeric state and homogeneity, while circular dichroism (CD) can analyze secondary structure content and thermal stability. These studies provide critical data to answer the question of whether the protein is correctly folded and monodisperse. The outcomes directly determine the suitability for any functional application (like Application 1).
4. Comparative Proteomics and Mass Spectrometry Analysis
This recombinant msyB protein is ideal as a quantitative standard for mass spectrometry. Its defined sequence and purity allow it to be used to generate standard curves for absolute quantification (e.g., using AQUA peptides) or to optimize LC-MS/MS parameters for detecting endogenous msyB in complex E. coli lysates.
Final Recommendation & Action Plan
The unknown folding state mandates a sequential, risk-averse approach. The immediate and mandatory first step is to prioritize Application 3 (Biochemical Characterization) to assess the protein's folding integrity and homogeneity via SEC and CD. If it is well-folded and monodisperse, it may then be considered for Application 1 (Interaction Studies), though a positive control interaction should be tested to confirm functional competence. Regardless of the folding results, Applications 2 and 4 (Antibody Development and Mass Spectrometry) can proceed immediately and confidently, as they do not require a natively folded structure. Investing in interaction studies before biophysical validation is a high-risk strategy likely to yield uninterpretable results.