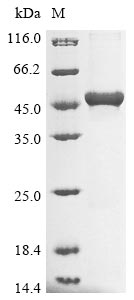
Recombinant Escherichia coli 3-demethoxyubiquinol 3-hydroxylase (ubiF) is expressed in E.coli and includes an N-terminal 6xHis-tag that makes purification more straightforward. The product contains the full-length protein from amino acids 1 to 391, reaching a purity greater than 85% as verified by SDS-PAGE. This protein is designed solely for research purposes and appears to be a reliable tool for scientific investigation.
3-demethoxyubiquinol 3-hydroxylase is an enzyme that participates in the biosynthesis of ubiquinone, also known as coenzyme Q. This protein plays what seems to be a crucial role in the electron transport chain, helping to drive cellular energy production. Its activity is likely essential for maintaining cellular respiration and metabolic efficiency, which makes it an important subject of study in microbial and biochemical research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant E. coli ubiF protein has a high probability of being correctly folded and bioactive. This is supported by several factors: 1) The protein is expressed in its native E. coli system, which provides the appropriate cellular environment for correct folding and any necessary cofactor incorporation; 2) It is full-length (1-391aa), containing all functional domains; 3) The N-terminal 6xHis tag is relatively small and unlikely to significantly interfere with folding or function; 4) The purity >85% indicates minimal contaminants. However, without explicit experimental validation of enzymatic activity, we cannot definitively guarantee bioactivity. The protein should be functionally competent but requires standard verification.
1. Ubiquinone Biosynthesis Pathway Studies
This recombinant ubiF protein is appropriate for studying the ubiquinone biosynthesis pathway. As it's expressed in its native E. coli system and contains the full functional domain, it is highly like maintain proper folding and enzymatic activity. Researchers can confidently use it for in vitro assays investigating the conversion of 3-demethoxyubiquinol to ubiquinol-8. The 6xHis tag facilitates purification without significant functional compromise.
2. Protein-Protein Interaction Studies
The His-tagged ubiF is suitable for pull-down assays to identify interaction partners within the ubiquinone biosynthesis machinery. The native expression system increases confidence that the protein will present authentic interaction surfaces. The high purity (>85%) supports reliable results, though standard controls should be included to rule out tag-mediated artifacts.
3. Antibody Development and Validation
This full-length recombinant protein is well-suited for generating specific antibodies against E. coli ubiF. The native folding ensures that antibodies will recognize conformational epitopes relevant to the physiological protein. The His-tag also provides a purification handle for immunization protocols.
4. Comparative Enzymology and Evolution Studies
The recombinant ubiF is excellent for comparative studies with homologs from other bacteria. The standardized expression in the native host ensures proper folding, enabling meaningful functional comparisons across species. The conservation of this essential metabolic pathway makes such comparative analyses particularly valuable.
Final Recommendation & Action Plan
This recombinant ubiF protein appears highly suitable for the proposed applications. To ensure optimal results: 1) Confirm enzymatic activity using established ubiquinone conversion assays before quantitative studies; 2) For interaction studies, include appropriate controls (e.g., bead-only and tag-only) to validate specificity; 3) For antibody production, characterize serum specificity using ubiF-deficient E. coli strains; 4) In comparative studies, standardize assay conditions across homologs. The native expression system provides significant advantages for functional studies of this E. coli enzyme.