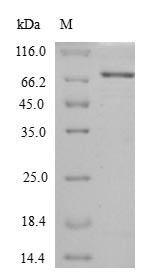
Recombinant Escherichia coli Chaperone protein HtpG comes from an E. coli expression system and includes the complete protein sequence from amino acids 1 to 624. The preparation contains no tags, which appears to minimize potential interference with normal protein function. SDS-PAGE analysis confirms purity levels above 90%, and the product shows no detectable endotoxins—both factors that may contribute to more reliable experimental outcomes.
HtpG belongs to the Hsp90 chaperone family and seems to play an essential role in how E. coli manages protein folding and stabilization. The protein likely helps newly synthesized polypeptides fold correctly and may assist in refolding proteins that become damaged under stress conditions. Research suggests HtpG is particularly important when bacteria face environmental challenges, which makes it an interesting target for studying how microorganisms respond to stress and maintain protein balance.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
E. coli HtpG is a bacterial homolog of Hsp90 that functions as a molecular chaperone, requiring ATP binding/hydrolysis and proper dimerization for activity. The E. coli expression system is homologous for this protein, significantly increasing the probability of correct folding. As a chaperone protein itself, HtpG has inherent stability and folding capacity. The full-length sequence (1-624aa) contains all necessary domains for chaperone activity. Therefore, this recombinant HtpG has a high probability of being correctly folded and functionally active.
1. Protein-Protein Interaction Studies
This recombinant HtpG is highly suitable for studying interactions with client proteins and co-chaperones. The tag-free, full-length protein preserves all native interaction surfaces. Techniques like pull-down assays and surface plasmon resonance can reliably identify physiological binding partners involved in protein folding and stress response pathways. Any interaction data would be biologically irrelevant without validation using natively folded protein.
2. Biochemical Characterization and ATPase Activity Assays
This protein is suitable for detailed biochemical analysis including ATPase activity measurements, nucleotide binding studies, and kinetic parameter determination. The high purity (>90%) ensures accurate measurements without interference from contaminants. ATPase assays will provide direct functional validation of chaperone activity.
3. Antibody Development and Validation
This recombinant HtpG serves as an excellent immunogen for generating specific antibodies against E. coli HtpG. The full-length, tag-free protein presents all native epitopes, ensuring antibodies will recognize the physiological form of the chaperone. The high purity minimizes antibodies against contaminants.
4. Protein Folding and Stability Studies
This protein is well-suited for biophysical characterization of chaperone stability using techniques like differential scanning calorimetry and circular dichroism spectroscopy. As a chaperone itself, studying HtpG's folding stability provides insights into its functional mechanism under various stress conditions.
Final Recommendation & Action Plan
This recombinant HtpG expressed in its native E. coli system without fusion tags is highly likely to be properly folded and functionally active, making it suitable for all proposed applications. The recommended approach is to begin with Application 2 (Biochemical Characterization) to confirm ATPase activity and validate functional competence. Once activity is verified, proceed confidently with Applications 1, 3, and 4 for interaction studies, antibody development, and stability analyses. The tag-free, full-length design in a homologous expression system provides optimal conditions for biologically relevant results across all experimental paradigms.