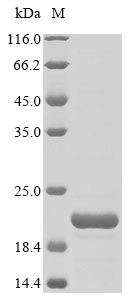
The first step in producing the recombinant Griffithsia sp. Griffithsin protein is to construct a plasmid that contains the Griffithsia sp. Griffithsin protein (1-121aa(X31S)) encoding gene along with the N-terminal 10xHis-tag gene and C-terminal Myc-tag gene. The next is to transform this plasmid into E.coli cells and select positive E.coli cells, from which positive cells can be screened and cultured to express the protein. The recombinant Griffithsia sp. Griffithsin protein is purified through affinity purification from the cell lysate. Its purity is greater than 85%, determined by the SDS-PAGE analysis.
Griffithsin (GRFT) is a 121 amino acid lectin derived from the red marine alga Griffithsia sp. It is a broad-spectrum antiviral protein that has shown potent activity against various glycosylated viruses, including HIV, hepatitis C virus, SARS coronavirus, Japanese encephalitis virus, and herpes simplex virus [1][2][3]. Griffithsin exhibits its antiviral activity by binding to oligomannose glycans found on viral envelope glycoproteins, thereby preventing viral entry and infection [4][5]. The lectin has been found to have a unique structure with six independent sugar binding sites, allowing it to bind to multiple individual sugars on large oligosaccharides, such as Man9GlcNAc2 [4]. This ability enables Griffithsin to interact with various enveloped viruses effectively.
Moreover, Griffithsin has been identified as a potent inhibitor of viruses like HCV, SARS coronavirus, Middle East respiratory syndrome coronavirus, and herpes simplex virus, both in vitro and in vivo, while demonstrating minimal toxicity [3]. Lectin has also been suggested as a promising candidate for microbicide development due to its high mannose-targeting properties and potential to prevent viral infections [6][7]. Additionally, Griffithsin has been explored for large-scale production using plant-based systems like Nicotiana excelsiana, indicating its potential for cost-effective manufacturing [8][9].
References:
[1] H. Ishag, C. Li, L. Huang, M. Sun, F. Wang, B. Niet al., Griffithsin inhibits japanese encephalitis virus infection in vitro and in vivo, Archives of Virology, vol. 158, no. 2, p. 349-358, 2012. https://doi.org/10.1007/s00705-012-1489-2
[2] J. Xue, B. Hoorelbeke, I. Kagiampakis, B. Demeler, J. Balzarini, & P. LiWang, The griffithsin dimer is required for high-potency inhibition of hiv-1: evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism, Antimicrobial Agents and Chemotherapy, vol. 57, no. 8, p. 3976-3989, 2013. https://doi.org/10.1128/aac.00332-13
[3] J. Millet, K. Séron, R. Labitt, A. Danneels, K. Palmer, G. Whittakeret al., Middle east respiratory syndrome coronavirus infection is inhibited by griffithsin, Antiviral Research, vol. 133, p. 1-8, 2016. https://doi.org/10.1016/j.antiviral.2016.07.011
[4] N. Ziółkowska, S. Shenoy, B. O’Keefe, J. McMahon, K. Palmer, R. Dweket al., Crystallographic, thermodynamic, and molecular modeling studies of the mode of binding of oligosaccharides to the potent antiviral protein griffithsin, Proteins Structure Function and Bioinformatics, vol. 67, no. 3, p. 661-670, 2007. https://doi.org/10.1002/prot.21336
[5] A. Alam, L. Jiang, G. Kittleson, K. Steadman, S. Nandi, J. Fuquaet al., Technoeconomic modeling of plant-based griffithsin manufacturing, Frontiers in Bioengineering and Biotechnology, vol. 6, 2018. https://doi.org/10.3389/fbioe.2018.00102
[6] J. Fuqua, V. Wanga, & K. Palmer, Improving the large scale purification of the hiv microbicide, griffithsin, BMC Biotechnology, vol. 15, no. 1, 2015. https://doi.org/10.1186/s12896-015-0120-5
[7] N. Ziółkowska, B. O'Keefe, T. Mori, C. Zhu, B. Giomarelli, F. Vojdaniet al., Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding, Structure, vol. 14, no. 7, p. 1127-1135, 2006. https://doi.org/10.1016/j.str.2006.05.017
[8] P. Eapen, J. Cates, R. Mundell, K. Palmer, & J. Fuqua, In preparation for outdoor pharming: griffithsin can be expressed in nicotiana excelsiana and retains activity after storage as silage, Frontiers in Bioengineering and Biotechnology, vol. 8, 2020. https://doi.org/10.3389/fbioe.2020.00199
[9] J. Decker, R. Menacho‐Melgar, & M. Lynch, Low-cost, large-scale production of the anti-viral lectin griffithsin, Frontiers in Bioengineering and Biotechnology, vol. 8, 2020. https://doi.org/10.3389/fbioe.2020.01020