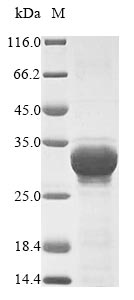
Recombinant Hordeum vulgare Oxalate oxidase 1 production in E. coli involves several stages. Initially, the gene encoding the full-length Hordeum vulgare Oxalate oxidase 1 (1-201aa) is co-cloned into an expression vector with an N-terminal 10xHis-tag and C-terminal Myc-tag gene and transformed into E. coli cells. The bacteria are grown under conditions that induce protein expression. After sufficient growth, the cells are lysed to release the recombinant protein. The collected proteins are subjected to affinity chromatography purification. Protein purity is assessed using SDS-PAGE, exceeding 90%.
Hordeum vulgare Oxalate oxidase 1 (OXO1) is an enzyme that plays a crucial role in degrading oxalate into carbon dioxide and hydrogen peroxide [1]. This enzyme has been characterized in barley, and its biochemical functions are still being further investigated [1].
Research has shown that Hordeum vulgare OXO1 requires manganese for its catalytic activity, and its crystal structure reveals specific binding motifs for this metal ion [2]. Furthermore, Hordeum vulgare OXO1 has been associated with plant defense mechanisms, as its activity increases in barley and wheat leaves following infection with the powdery mildew fungus [3]. This enzyme's importance in plant-pathogen interactions highlights its role in plant immunity and defense responses.
Moreover, HvOXO1 has been linked to biotransformation processes in barley cells, demonstrating oxidase and epimerase activities in converting compounds like podophyllotoxin into isopicropodophyllone [4][5]. The enzyme's involvement in these biotransformation pathways showcases its versatility and significance in plant metabolism.
References:
[1] B. Dumas, G. Freyssinet, & K. Pallett, Tissue-specific expression of germin-like oxalate oxidase during development and fungal infection of barley seedlings, Plant Physiology, vol. 107, no. 4, p. 1091-1096, 1995. https://doi.org/10.1104/pp.107.4.1091
[2] H. Pan, M. Whittaker, R. Bouveret, A. Berna, F. Bernier, & J. Whittaker, Characterization of wheat germin (oxalate oxidase) expressed by pichia pastoris, Biochemical and Biophysical Research Communications, vol. 356, no. 4, p. 925-929, 2007. https://doi.org/10.1016/j.bbrc.2007.03.097
[3] F. Zhou, Z. Zhang, P. Gregersen, J. Mikkelsen, E. Neergaard, D. Collingeet al., Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus1, Plant Physiology, vol. 117, no. 1, p. 33-41, 1998. https://doi.org/10.1104/pp.117.1.33
[4] R. Teng, D. McManus, J. Aylward, S. Ogbourne, D. Armstrong, S. Mauet al., Biotransformation of ingenol-3-angelate in four plant cell suspension cultures, Biocatalysis and Biotransformation, vol. 27, no. 3, p. 186-194, 2009. https://doi.org/10.1080/10242420902811105
[5] R. Teng, D. McManus, S. Mau, & A. Bacic, Biotransformation of podophyllotoxin byhordeum vulgarecell suspension cultures, Biocatalysis and Biotransformation, vol. 25, no. 1, p. 1-8, 2007. https://doi.org/10.1080/10242420601051201