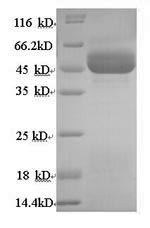
Recombinant Human 11-beta-hydroxysteroid dehydrogenase type 2 (HSD11B2) is produced in yeast and contains the complete protein sequence (1-405 amino acids) with an N-terminal 6xHis-tag that makes purification straightforward. SDS-PAGE analysis confirms the protein reaches greater than 90% purity, which appears to make it well-suited for research applications that demand high-quality reagents.
HSD11B2 serves a key function in glucocorticoid metabolism. It converts active cortisol into its inactive form, cortisone. The enzyme seems particularly important for maintaining mineralocorticoid receptor specificity—it does this by regulating local cortisol concentrations, which in turn affects sodium balance and blood pressure control. Research into this enzyme may prove essential for understanding corticosteroid-related pathways and associated disorders.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the folding state and bioactivity of this recombinant HSD11B2 protein are unknown and must be considered highly uncertain. HSD11B2 is an enzyme that requires precise folding for its catalytic activity, correct binding of its NAD+ cofactor, and formation of its active site. While expression in a yeast system (a eukaryotic host) is more favorable than bacteria for producing complex eukaryotic proteins and may support disulfide bond formation, there is no guarantee of correct folding or activity. The presence of an N-terminal 6xHis tag could potentially interfere with the protein's N-terminal region or its oligomerization. The >90% purity indicates a clean preparation, but does not confirm a functional enzyme. Therefore, applications that depend on the protein's specific enzymatic activity or native conformation are speculative without validation.
1. Protein-Protein Interaction Studies Using His-Tag Pull-Down Assays
The N-terminal 6xHis-tag allows this recombinant HSD11B2 to be immobilized for pull-down experiments. However, the utility of these assays for identifying biological interaction partners is entirely contingent on the protein being correctly folded. If the protein is misfolded and inactive, it will not present the correct surfaces for specific interactions with cofactors or regulatory proteins. Any interactions identified could be non-specific artifacts of the misfolded state. This approach is not recommended for elucidating regulatory mechanisms without first confirming the protein's native structure.
2. Antibody Development and Validation
This full-length recombinant HSD11B2 protein is suitable for use as an immunogen to generate antibodies. The high purity is adequate for immunization. However, it is critical to understand that antibodies generated will be primarily against the linear epitopes of this recombinant HSD11B2 protein. Their ability to recognize the natively folded, active HSD11B2 enzyme in human tissues is not guaranteed and must be empirically validated. The protein is reliable for developing antibodies for techniques like Western blotting, but its suitability for functional studies (e.g., blocking activity) is uncertain.
3. Biochemical Characterization and Cofactor Binding Studies
This recombinant HSD11B2 protein is a starting point for biochemical characterization. However, the entire premise of studying "cofactor requirements" and "binding studies with NAD+" is invalid without first confirming the protein's enzymatic activity. The logical first step is to perform an enzymatic activity assay. If active, then studies on cofactor kinetics and stability can be pursued meaningfully. If inactive, any binding data would be uninterpretable.
4. Structural Biology Applications for Crystallization Screening
The high purity and full-length nature of the recombinant HSD11B2 protein make it suitable for initial crystallization trials. However, success is entirely dependent on the protein being homogeneous and correctly folded. A misfolded HSD11B2 protein is highly unlikely to yield diffractable crystals. The His-tag can be used for purification but may need to be removed to facilitate crystallization, as flexible tags can hinder crystal packing. This application is a low-probability, high-reward endeavor that can be attempted but should not be the primary use without activity confirmation.
Final Recommendation & Action Plan
The immediate and essential first step is to experimentally validate the enzymatic activity of this recombinant HSD11B2 protein using a standard dehydrogenase activity assay (e.g., measuring the conversion of corticosterone to 11-dehydrocorticosterone). The outcome of this test is critical and dictates all subsequent applications: a positive result would justify and enable functional studies in Applications 1 (interactions), 3 (biochemistry/cofactor binding), and 4 (crystallography). A negative result would limit its reliable use to Application 2 (antibody production for linear epitopes) and would necessitate troubleshooting of the expression and purification protocol to obtain an active protein. Therefore, the activity assay is a non-negotiable prerequisite before investing significant resources in most proposed applications.