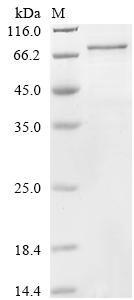
Recombinant Human Cysteine-rich secretory protein LCCL domain-containing 1 (CRISPLD1) is produced through an E.coli-based cell-free expression system, which appears to ensure high-quality synthesis of the full-length mature protein (amino acids 24-500). The product comes with an N-terminal GST tag that helps with purification and detection. SDS-PAGE verification shows purity exceeding 85%, making it what seems like a reliable reagent for research work. Endotoxin levels stay within industry standards, which should make it compatible with different experimental protocols.
CRISPLD1 is a protein that researchers believe plays a part in cell signaling pathways. It likely contributes to extracellular matrix organization - something that's pretty important for tissue development and repair. The structural domains suggest it might function in protein-protein interactions, which makes it an interesting target for studying how cells communicate and maintain tissue balance. Getting a better handle on CRISPLD1 could shed light on basic biological processes and what they mean for health and disease.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The human CRISPLD1 contains multiple functional domains, including the LCCL domain, which typically requires proper disulfide bond formation and correct folding for its suggested roles in innate immunity and extracellular matrix interactions. While cell-free systems can improve solubility compared to traditional E. coli expression, they still lack the eukaryotic folding machinery and may not support proper disulfide bond formation for complex multidomain proteins. The large GST tag (≈26 kDa) may sterically hinder proper folding of the CRISPLD1 domains. Without experimental validation (e.g., structural analysis or functional assays), the protein cannot be assumed to be correctly folded or bioactive.
1. GST Pull-Down Assays for Protein-Protein Interaction Studies
The GST tag enables pull-down assays to identify potential binding partners. However, if CRISPLD1 is misfolded, interactions may be non-physiological. The GST tag itself may cause non-specific binding. Validate any identified interactions using full-length CRISPLD1 expressed in mammalian cells or confirm with co-immunoprecipitation from native sources.
2. Antibody Development and Validation
This application is suitable. The recombinant CRISPLD1 can serve as an immunogen for generating antibodies against linear epitopes, even if misfolded. The high purity supports consistent immunization. However, antibodies generated may not recognize conformational epitopes of native, properly folded CRISPLD1. Validate antibody specificity against endogenous CRISPLD1 from human cell lysates or tissues.
3. Biochemical Characterization and Protein Stability Studies
It is suitable for basic biophysical analysis of the GST-tagged protein (e.g., thermal stability, aggregation state). However, data may not reflect native CRISPLD1 properties due to potential misfolding and the large GST tag. Use techniques like circular dichroism to assess secondary structure, but interpret results cautiously without native CRISPLD1 controls.
4. ELISA-Based Quantitative Assays
Feasible for developing ELISA assays, but if CRISPLD1 is misfolded, the assay may not accurately quantify native CRISPLD1 in biological samples. The GST tag may block epitopes or affect antibody binding. Validate the ELISA with native CRISPLD1 standards to ensure correlation with physiological levels.
Final Recommendation & Action Plan
Before using this recombinant CRISPLD1 for functional studies, validate its folding and structural integrity. First, analyze secondary structure via circular dichroism and check for proper disulfide bond formation via non-reducing SDS-PAGE. If possible, test functionality through known binding interactions (e.g., with bacterial components or extracellular matrix proteins). For pull-down studies, consider cleaving the GST tag to reduce steric interference. Always validate findings with native CRISPLD1 from mammalian expression systems or endogenous sources. For reliable quantitative assays, develop standards using properly folded CRISPLD1.