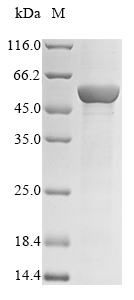
The Human CYP2C19 protein's gene (26-490aa) is inserted into a plasmid vector, forming recombinant plasmid, which is introduced into e.coli cells. e.coli cells surviving in the presence of a specific antibiotic are selected and then cultured under conditions promoting the expression of the gene of interest. The protein features a N-terminal 10xHis tag and C-terminal Myc tag fusion. After expression, the recombinant Human CYP2C19 protein is isolated and purified from the cell lysate through affinity purification. Denaturing SDS-PAGE is utilized to resolve the resulting recombinant protein, revealing a purity exceeding 85%.