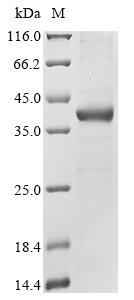
Recombinant Human Epididymal secretory glutathione peroxidase (GPX5) is produced in E. coli and features an N-terminal 6xHis-GST tag that makes purification straightforward. This full-length Isoform 2 protein contains amino acids 1-100 and shows a purity level greater than 85% as determined by SDS-PAGE. This product is intended for research use only and is not suitable for diagnostic or therapeutic applications.
Epididymal secretory glutathione peroxidase (GPX5) is an antioxidant enzyme that appears to play a crucial role in protecting cells from oxidative stress by reducing lipid hydroperoxides and hydrogen peroxide. It belongs to the glutathione peroxidase family, which seems integral to cellular defense mechanisms against reactive oxygen species. GPX5 may be particularly relevant in reproductive biology, where it's involved in maintaining the integrity of sperm cells.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant human GPX5 protein is unlikely to be correctly folded or bioactive. Several critical factors contribute to this assessment: 1) GPX5 is a selenocysteine-containing enzyme that requires specific translational machinery for proper incorporation of selenocysteine at the active site - this cannot be achieved in standard E. coli expression systems; 2) The N-terminal 6xHis-GST tag is exceptionally large (~26 kDa for GST plus His-tag) compared to the GPX5 domain (∼11 kDa, 1-100aa), which will likely cause severe steric hindrance and prevent proper folding; 3) The protein represents a truncated form (1-100aa) that may lack critical C-terminal regions necessary for full enzymatic activity; 4) Glutathione peroxidases require specific redox conditions for proper disulfide bond formation and active site configuration that E. coli may not provide. The >85% purity indicates minimal contaminants, but doesn't overcome these fundamental limitations.
This recombinant protein can generate antibodies, but they will primarily recognize the immunodominant GST tag rather than GPX5-specific epitopes. If the protein is misfolded (likely due to the expression system and large tag), antibodies may not recognize native GPX5. For specific antibodies, use properly folded, tag-free GPX5 expressed in a eukaryotic system and validate against the endogenous protein.
This recombinant GPX5 has fundamental limitations that preclude its use for most proposed applications. For reliable results: 1) Use a eukaryotic expression system (mammalian or insect cells) that supports selenocysteine incorporation for any enzymatic or functional studies; 2) Remove the GST tag and use only a small affinity tag for structural and interaction studies; 3) Use full-length GPX5 rather than the truncated form; 4) Always include positive controls with known enzymatic activity when testing GPX function. For antibody production, consider alternative antigens or validate extensively against native GPX5. The current protein may only be suitable for tag-specific antibody generation or as a negative control in limited applications.