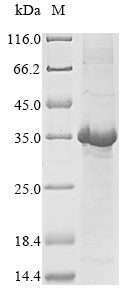
The expression region of this recombinant Human DEFA1 covers amino acids 1-94. This DEFA1 protein is theoretically predicted to have a molecular weight of 37.2 kDa. Expression of this DEFA1 protein is conducted in e.coli. The DEFA1 gene fragment has been modified by fusing the N-terminal GST tag, providing convenience in detecting and purifying the recombinant DEFA1 protein during the following stages.
Neutrophil defensin 1 (DEFA1) is a member of the defensin family, small cationic peptides with antimicrobial properties. DEFA1 is primarily produced by neutrophils, a type of white blood cell, and serves as part of the innate immune system's defense against microbial infections. This antimicrobial peptide exerts its function by disrupting the microbial cell membrane, leading to cell death. Beyond its antimicrobial role, DEFA1 has been implicated in various immune responses and inflammatory processes. Research areas related to DEFA1 include investigations into its role in host-pathogen interactions, immune regulation, and potential therapeutic applications in combating infectious diseases. Understanding the functions of DEFA1 contributes to insights into the immune system's defense mechanisms.