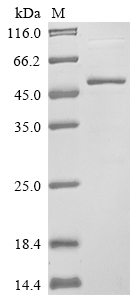
Recombinant Human Protein unc-13 homolog A (UNC13A) gets expressed in E.coli, covering the 1-340 amino acid region, with an N-terminal 6xHis tag attached for simpler purification. The protein comes at a purity level greater than 85% as confirmed by SDS-PAGE analysis, which appears to provide adequate reliability for research applications. This product is meant for research use only and doesn't include functional or disease relevance data.
UNC13A is a protein that seems to play a key role in synaptic vesicle maturation and neurotransmitter release. It likely functions as a critical component of the presynaptic machinery, contributing to the regulation of synaptic plasticity and neuronal communication. Researchers find UNC13A valuable for its involvement in understanding synaptic function and exploring neurological pathways, making it an important target in neuroscience studies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Human UNC13A (a eukaryotic neuronal protein involved in synaptic vesicle priming) is expressed in E. coli, a prokaryotic system that lacks the machinery for proper folding of complex eukaryotic proteins. UNC13A contains multiple domains (C1, C2, MUN) that require precise folding and potentially post-translational modifications for functionality. The expressed fragment (1-340aa) is partial and may lack critical functional domains. The N-terminal 6xHis tag could further interfere with native folding. Since activity is unverified and the expression system is mismatched for this complex neuronal protein, the recombinant protein is unlikely to be correctly folded or bioactive without experimental validation.
1. Protein-Protein Interaction Studies Using His-Tag Pull-Down Assays
While technically feasible due to the His-tag, this application is high-risk without folding validation. If UNC13A is misfolded (as is likely), it may not interact authentically with biological partners, leading to the identification of non-physiological interactions. The partial sequence (1-340aa) may contain some interaction domains, but cannot represent full UNC13A functionality. Results would require confirmation with a full-length, properly folded protein.
2. Antibody Development and Validation
This application is appropriate. The recombinant fragment can serve as an immunogen for generating antibodies targeting linear epitopes in the N-terminal region (1-340aa) of UNC13A. The >85% purity is sufficient for immunization, and the His-tag facilitates purification. However, antibodies may not recognize conformational epitopes or the full-length native protein without additional validation.
3. Biochemical Characterization and Stability Studies
This is a valuable and appropriate application. Biophysical techniques (dynamic light scattering, differential scanning fluorimetry) can directly assess the protein's folding state, stability, and aggregation propensity. These studies are essential for characterizing the recombinant product itself, regardless of its native functionality, and can inform whether further purification or refolding is needed.
4. Structural Biology Sample Preparation and Optimization
This application is suitable for method development but has limitations. The protein can be used to optimize expression and purification protocols. However, the >85% purity is insufficient for actual structural studies, and the likelihood of misfolding makes it problematic for structural biology applications requiring native conformation. It's more appropriate for developing technical protocols rather than structural analysis.
Final Recommendation & Action Plan
Given the high probability of misfolding in E. coli expression, recommend first conducting biophysical characterization (Application #3) to assess the protein's folding state and stability. If the protein shows monodisperse behavior and expected secondary structure, it could be used cautiously for interaction studies (with appropriate controls) and antibody development. For structural studies, significantly higher purity (>95%) and folding validation would be required. Given the limitations of prokaryotic expression for complex neuronal proteins, consider alternative expression systems (e.g., insect or mammalian cells) for functional studies requiring properly folded UNC13A.