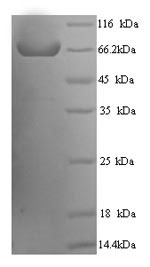
Recombinant human receptor-type tyrosine-protein phosphatase N2 (PTPRN2) production in yeast starts with co-inserting the gene encoding the extracellular domain of the PTPRN2 protein (22-615aa) into an expression vector with an N-terminal 6xHis-tag gene, followed by transformation into yeast cells. The cells are cultured under conditions that promote protein expression. Once sufficient growth is achieved, the cells are lysed to release the recombinant PTPRN2 protein. Purification is achieved using affinity chromatography. The purity of the recombinant protein is confirmed using SDS-PAGE, exceeding 90%.
PTPRN2 is a tyrosine phosphatase involved in dephosphorylating phosphoinositols and is identified as an autoantigen in type 1 diabetes.
It is associated with insulin regulation and secretion [1][2]. PTPRN2 has been linked to childhood obesity through genetically controlled CpG methylation [3]. Studies have shown that DNA methylation in human islets, mediated by PTPRN2, may influence insulin secretion and contribute to the pathogenesis of type 2 diabetes [4]. Furthermore, PTPRN2 has been associated with differential DNA methylation and gene expression in human placentas, impacting vascular and metabolic diseases [5]. PTPRN2 also regulates insulin secretion, which is crucial for puberty onset [6],
PTPRN2 has also been implicated in promoting malignant transformation in colorectal cancer cells through the EMT/TRAF2/STAT3 signaling pathway [7]. Furthermore, PTPRN2 has been related to metastatic breast cancer cell migration through PI(4,5)P2-dependent actin remodeling [8].
References:
[1] J. Kulski, Long noncoding rna hcp5, a hybrid hla class i endogenous retroviral gene: structure, expression, and disease associations, Cells, vol. 8, no. 5, p. 480, 2019. https://doi.org/10.3390/cells8050480
[2] C. Ling, Epigenetic regulation of insulin action and secretion – role in the pathogenesis of type 2 diabetes, Journal of Internal Medicine, vol. 288, no. 2, p. 158-167, 2020. https://doi.org/10.1111/joim.13049
[3] S. Lee, The association of genetically controlled cpg methylation (cg158269415) of protein tyrosine phosphatase, receptor type n2 (ptprn2) with childhood obesity, Scientific Reports, vol. 9, no. 1, 2019. https://doi.org/10.1038/s41598-019-40486-w
[4] P. Chen, A. Chu, W. Liao, L. Rubbi, C. Janzen, F. Hsuet al., Prenatal growth patterns and birthweight are associated with differential dna methylation and gene expression of cardiometabolic risk genes in human placentas: a discovery-based approach, Reproductive Sciences, vol. 25, no. 4, p. 523-539, 2018. https://doi.org/10.1177/1933719117716779
[5] C. Yang, J. Ye, Y. Liu, J. Ding, H. Liu, X. Gaoet al., Methylation pattern variation between goats and rats during the onset of puberty, Reproduction in Domestic Animals, vol. 53, no. 3, p. 793-800, 2018. https://doi.org/10.1111/rda.13172
[6] K. Lim, P. Jenjaroenpun, Z. Low, Z. Khong, Y. Ng, V. Kuznetsovet al., Duplex stem-loop-containing quadruplex motifs in the human genome: a combined genomic and structural study, Nucleic Acids Research, vol. 43, no. 11, p. 5630-5646, 2015. https://doi.org/10.1093/nar/gkv355
[7] J. Chen, Aberrant expression of ptprn2 promotes malignant transformation of colorectal cancer cells through emt/traf2/stat3 signaling pathway,, 2023. https://doi.org/10.21203/rs.3.rs-3155307/v1
[8] R. Fernández-Carrión, J. Sorlí, O. Coltell, E. Pascual, C. Ortega, R. Barragánet al., Sweet taste preference: relationships with other tastes, liking for sugary foods and exploratory genome-wide association analysis in subjects with metabolic syndrome, Biomedicines, vol. 10, no. 1, p. 79, 2021. https://doi.org/10.3390/biomedicines10010079