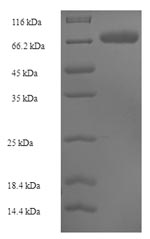
The region for expressing recombinant Human SUOX contains amino acids 80-545. This SUOX protein is expected to have a theoretical molecular weight of 67.6 kDa. The SUOX protein was expressed in e.coli. The SUOX coding gene included the N-terminal 6xHis-SUMO tag, which simplifies the detection and purification processes of the recombinant SUOX protein in following stages of expression and purification.
Sulfite oxidase, mitochondrial (SUOX) is a crucial enzyme involved in the oxidation of sulfite to sulfate in the mitochondria. This process is vital for the metabolism of sulfur-containing amino acids, such as cysteine and methionine. SUOX plays a pivotal role in maintaining sulfur homeostasis and preventing the accumulation of toxic sulfite levels in the body. Additionally, SUOX is essential for the proper functioning of the molybdenum cofactor (Moco), a prosthetic group required for the enzyme's activity. Mutations in the SUOX gene can lead to a rare inherited disorder known as isolated sulfite oxidase deficiency, characterized by neurological abnormalities and other health complications. Research on SUOX contributes to a better understanding of sulfur metabolism, redox balance, and the molecular basis of genetic disorders related to sulfite oxidase deficiency.