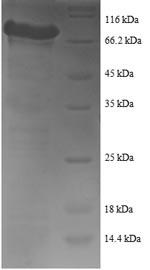
Recombination of a plasmid encoding the Human YARS1 protein (2-528aa) is the first step during the production the recombinant Human YARS1 protein. The constructed plasmid is introduced into e.coli cells. e.coli cells that can survive in the presence of a specific antibiotic are selected to be cultured for the induction of protein expression. The protein is equipped with a N-terminal GST tag. After expression, affinity purification is used to isolate and purify the recombinant Human YARS1 protein from the cell lysate. Denaturing SDS-PAGE is then applied to resolve the resulting recombinant Human YARS1 protein. Its purity exceeds 90%.