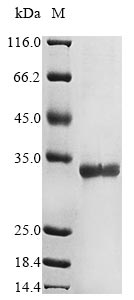
Recombinant Human herpesvirus 6B mRNA export factor ICP27 homolog (KA3L) is produced using an E. coli expression system. This partial protein covers amino acids 135 to 364 and comes with an N-terminal 10xHis tag and a C-terminal Myc tag. SDS-PAGE analysis shows the protein reaches greater than 85% purity, which appears to be sufficient for most research applications.
Human herpesvirus 6B's KA3L protein seems to play a crucial role in how the virus manages mRNA export—a process that's essential for viral replication and gene expression. Studying this protein may be important in virology research since it could provide insights into viral infection mechanisms and potentially inform therapeutic strategies against herpesvirus-related diseases.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Because KA3L is a viral regulatory protein—likely containing intrinsically disordered regions and requiring eukaryotic chaperones or post-translational modifications for proper folding—the E. coli expression system is unlikely to reproduce its native conformation or activity. The protein fragment (135–364aa) represents only part of the full-length protein, which further reduces the likelihood of maintaining the natural tertiary structure or biological function. Thus, this recombinant KA3L fragment is probably not correctly folded or functionally active, but it can still retain linear epitopes useful for antibody development or serve as a partial structural domain for limited biophysical characterization.
1. Antibody Development and Validation
The dual-tagged recombinant KA3L fragment is suitable for generating antibodies, particularly those targeting linear or partially structured epitopes within residues 135–364. Although the fragment is unlikely to fold natively, it can still elicit specific immune responses recognizing this protein region. The Myc-tag can assist in initial screening, while the His-tag allows for easy capture in ELISA or Western blot validation. However, antibodies should be tested against full-length KA3L expressed in eukaryotic systems to confirm their applicability in biological studies.
2. Biochemical Characterization and Structural Studies
The purified KA3L fragment can be used for basic biophysical characterization—such as circular dichroism or dynamic light scattering—to assess secondary structure content or aggregation state. However, due to its partial length and bacterial origin, this protein is unlikely to represent the native tertiary structure of KA3L. Such studies can still provide insight into intrinsic disorder or domain stability, but should not be interpreted as evidence of native folding or function.
3. Comparative Functional Analysis with Related Viral Proteins
The recombinant fragment can be used for sequence-level or antibody-based comparisons with homologous viral proteins (e.g., HSV-1 ICP27), but not for direct functional assays, as its activity is unlikely to be preserved. To perform meaningful comparative functional analyses, full-length and eukaryotically expressed constructs should be employed.
Final Recommendation & Action Plan
The recombinant HHV-6B KA3L fragment (135–364aa) expressed in E. coli is unlikely to be correctly folded or biologically active, as viral mRNA export factors typically require eukaryotic expression machinery for proper conformation and cofactor interactions. Nevertheless, this fragment can be reliably used for antibody generation, Western blot antigen standards, and preliminary biophysical studies focusing on linear epitope characterization or intrinsic disorder analysis. Avoid interpreting any binding or enzymatic data as physiologically relevant unless confirmed using full-length KA3L expressed in a eukaryotic system.