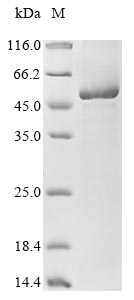
Recombinant Methanosarcina barkeri Pyrrolysine--tRNA ligase (pylS) is expressed in E.coli as a full-length protein comprising amino acids 1 to 419, with an N-terminal 6xHis tag for simplified purification. The protein reaches purity levels above 85% as determined by SDS-PAGE, which appears to ensure dependable performance in research applications. This product is suitable for research use only, not for therapeutic or diagnostic purposes.
Pyrrolysine--tRNA ligase, encoded by the pylS gene, plays a critical role in translation machinery by helping incorporate the rare amino acid pyrrolysine into proteins. This enzyme participates in a unique genetic code expansion process and seems essential for studying protein synthesis and engineering. Its function may be key to understanding how genetic code diversity and protein translation work in archaeal species.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Methanosarcina barkeri pylS is an archaeal enzyme that requires precise folding, ATP binding, and specific recognition of both pyrrolysine and its cognate tRNA for functional aminoacylation activity. While E. coli is a bacterial system and may not fully replicate archaeal-specific folding requirements, pylS has been successfully expressed in E. coli in numerous studies for genetic code expansion applications. The N-terminal 6xHis tag is small and unlikely to significantly interfere with the enzyme's functional domains. However, experimental validation remains essential to confirm functional activity in this specific preparation.
1. In Vitro Aminoacylation Assays for Pyrrolysine tRNA Charging
This application is highly dependent on proper folding and functional validation. Aminoacylation activity requires precise tertiary structure formation for substrate recognition and catalysis. If correctly folded and active (verified), the protein is excellent for studying tRNA charging kinetics and specificity. If misfolded/inactive (unverified), aminoacylation assays will yield negative results, providing no functional data.
2. Antibody Development and Immunological Studies
Antibody development relies on antigenic sequence recognition rather than functional folding. If correctly folded (verified), the protein excels for generating conformation-sensitive antibodies. If misfolded/unverified, it remains suitable for producing antibodies against linear epitopes of pylS.
3. Protein-Protein Interaction Studies Using Affinity Purification
This application requires proper folding validation. Protein-protein interactions within the pyrrolysine incorporation machinery require precise tertiary structure. If correctly folded (verified), the protein can identify physiological interaction partners. If misfolded/unverified, there is a high risk of non-specific binding or interaction failure.
4. Comparative Biochemical Analysis of Archaeal Translation Machinery
Meaningful comparative studies require native enzyme activity. If correctly folded and active (verified), the protein enables valid functional comparisons with other aminoacyl-tRNA synthetases. If misfolded/inactive (unverified), comparative analyses would yield misleading results about evolutionary relationships.
Final Recommendation & Action Plan
While E. coli expression has been used successfully for pylS in genetic code expansion studies, experimental validation of enzymatic activity is essential before reliable use in functional applications. Begin with biochemical characterization and activity assays to validate pyrrolysine-dependent tRNA charging activity using appropriate tRNA substrates and ATP. Once functional activity is verified, proceed confidently with Applications 1, 3, and 4 for aminoacylation studies, interaction mapping, and comparative analyses. Application 2 (antibody development) can proceed immediately regardless of folding status. If the protein is inactive, limit applications to linear epitope antibody production and basic biophysical characterization, avoiding all functional studies. For reliable pylS research, always include positive controls with known active pylS preparations and validate activity with functional tRNA charging assays before proceeding to downstream applications.