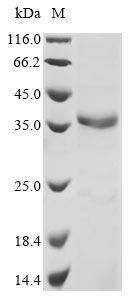
Recombinant Mouse ADAM DEC1 (Adamdec1) is expressed in E. coli and contains the full length of the mature protein, spanning amino acids 209 to 467. The protein comes with an N-terminal 6xHis-tag that helps with purification and detection. SDS-PAGE analysis confirms the product's purity exceeds 90%, which appears to make it suitable for various research applications.
ADAM DEC1 belongs to the ADAM (a disintegrin and metalloprotease) protein family. These proteins seem to be involved in several cellular processes, including cell signaling and adhesion. This particular protein likely plays an important role in regulating extracellular matrix composition and may be significant in studies related to tissue remodeling and repair. Understanding how it works could be crucial for advancing research in cell biology and related fields.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse ADAM DEC1 is a metalloprotease-domain containing protein that requires precise folding, disulfide bond formation, and proper zinc coordination for its functional activity. The E. coli expression system cannot provide the necessary eukaryotic post-translational modifications and complex folding environment required for this multidomain protein. While the protein may be soluble, it is highly unlikely to achieve the correct folding needed for functional protease activity or proper protein-protein interactions.
1. Antibody Development and Validation
This recombinant ADAM DEC1 serves as an excellent immunogen for generating antibodies against linear epitopes. The defined sequence (209-467aa) provides substantial epitope coverage for antibody production. However, antibodies may not efficiently recognize conformational epitopes on the native, properly folded protein.
2. Biochemical Characterization and Protein Stability Studies
These studies are essential for determining the protein's physical properties regardless of functional state. Basic biophysical characterization (size exclusion, thermal stability) can be performed, but functional protease assays will likely yield negative results due to probable misfolding.
3. ELISA-Based Quantitative Assays
Immunoassays rely on sequence-specific antibody recognition rather than native conformation. This protein is well-suited as a standard for quantitative immunoassays to detect immunoreactive ADAM DEC1. The assay depends on antibody binding to linear epitopes, making it reliable regardless of folding status. However, it will not measure functional protein levels.
Final Recommendation & Action Plan
The E. coli expression system is fundamentally unsuitable for producing a functional version of this complex metalloprotease-domain protein. The recombinant ADAM DEC1 is primarily suitable for antibody development and as an immunoassay standard, but requires rigorous validation before any functional applications. Begin with Application 2 (Biochemical Characterization) to assess basic physical properties through size-exclusion chromatography and circular dichroism spectroscopy. Applications 2 and 4 can proceed immediately for antibody production and ELISA development. Avoid interaction studies entirely due to the high probability of misfolding. For reliable ADAM DEC1 functional studies, use mammalian expression systems that support proper disulfide bond formation and domain folding.