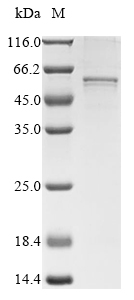
Recombinant Mouse Collagen alpha-1 (XXVI) chain (Col26a1) is expressed in a baculovirus system, spanning the full mature protein length from amino acids 21 to 440. The protein carries a 10xHis-tag at the N-terminus and a Myc-tag at the C-terminus for purification and detection purposes. SDS-PAGE analysis confirms that the preparation achieves greater than 85% purity, which appears suitable for research applications requiring high-quality protein.
Collagen alpha-1 (XXVI) chain represents a key component of the extracellular matrix, where it likely contributes to tissue structure and integrity. The protein seems to participate in pathways related to cell adhesion and intercellular communication. Studies of this protein may provide insights into tissue development and repair mechanisms, potentially making it valuable for developmental biology and regenerative medicine research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse Col26a1 is a type XXVI collagen that requires precise folding, proper triple helix formation, and specific post-translational modifications (particularly proline hydroxylation) for its structural function in the extracellular matrix. While the baculovirus-insect cell expression system provides a eukaryotic environment that supports proper protein folding and some post-translational modifications, it has significant limitations for producing functional collagen proteins due to incomplete post-translational modification machinery. Collagen proteins typically require specific hydroxylation enzymes and chaperones for correct triple helix formation that may not be fully present in insect cells. The dual N-terminal 10xHis-tag and C-terminal Myc-tag may significantly interfere with the protein's ability to form proper collagenous triple helices and interact with natural binding partners. While the expression system increases folding probability compared to prokaryotic systems, experimental validation is essential to confirm structural integrity and collagen functionality.
1. Collagen Structure-Function Analysis in Extracellular Matrix Research
This application carries significant limitations due to collagen's complex structural requirements. Proper Col26a1 function depends on triple helix formation and specific hydroxylation patterns that may not be fully replicated in baculovirus systems. If correctly folded with proper triple helix formation (verified), the protein may be suitable for basic structural studies. If misfolded/unverified (more likely), structural analyses will not reflect native collagen properties. The tags may prevent proper triple helix assembly and alter thermal stability measurements.
2. Antibody Development and Validation Studies
This application is highly suitable as antibody development primarily relies on linear epitope recognition rather than functional collagen structure. The mature protein region provides comprehensive epitope coverage for generating antibodies against Col26a1. The high purity ensures minimal contamination-related issues, and the dual tags facilitate efficient purification and screening processes.
Final Recommendation & Action Plan
The baculovirus expression system has significant limitations for producing functional collagen proteins due to incomplete post-translational modification machinery. This recombinant Col26a1 is primarily suitable for Application 2 (antibody development) but should be used with extreme caution for other applications. Begin with rigorous structural characterization, including circular dichroism spectroscopy to assess triple helix formation, thermal denaturation studies to evaluate stability, and mass spectrometry to verify hydroxylation patterns. If proper collagen structure is confirmed, limited Application 1 studies may be possible. Native collagen interactions with extracellular matrix components require precise triple helix conformation and presentation of binding domains. The tags will likely create artificial surfaces leading to non-specific binding, and the lack of proper hydroxylation and triple helix formation will prevent identification of genuine physiological interactions. For reliable collagen research requiring native structure and function, consider using mammalian expression systems that provide complete collagen modification machinery or work with collagen isolated from native tissues.