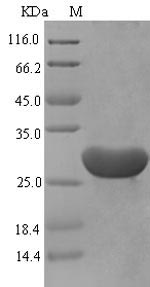
The production of recombinant mouse Serum amyloid A-1 protein (Saa1) in E. coli involves several steps. First, the gene encoding the full length of the mature Saa1 protein (20-122aa) is co-cloned into an expression vector with an N-terminal 6xHis-SUMO-tag gene and transformed into E. coli cells. The bacteria are cultured under conditions that induce protein expression. After sufficient growth, the cells are lysed to release the recombinant Saa1 protein. The recombinant Saa1 protein is purified by affinity chromatography. Protein purity is assessed using SDS-PAGE, reaching up to 90%.
Mouse Saa1 is a member of the serum amyloid A family of apolipoproteins, known for its role as a sensitive acute-phase high-density lipoprotein [1]. Saa1 is predominantly secreted by hepatocytes and is widely accepted as an accurate and sensitive indicator of inflammation [2][3]. It is involved in immunomodulation in both sterile and non-sterile inflammation, regulating inflammation and immunity [4][5]. Saa1 has been identified as a potential prediction biomarker for metastasis of hepatocellular carcinoma [6]. Additionally, Saa1 induces inflammation, proliferation, and cell death in activated hepatic stellate cells [7].
Saa1 plays a crucial role in various physiological processes. It is involved in extracellular matrix remodeling in the human amnion, with implications for fetal membrane rupture. Moreover, Saa1 participates in parturition by being synthesized de novo in the placenta. In the context of sepsis-induced lung injury, mouse bone marrow mesenchymal stem cells have been found to inhibit lung injury via exosomal Saa1.
References:
[1] M. Yu, X. Li, Q. Li, S. Mo, N. Yin, F. Hanet al., Saa1 increases nox4/ros production to promote lps-induced inflammation in vascular smooth muscle cells through activating p38mapk/nf-κb pathway, BMC Molecular and Cell Biology, vol. 20, no. 1, 2019. https://doi.org/10.1186/s12860-019-0197-0
[2] S. Siegmund, M. Schlosser, F. Schildberg, E. Seki, S. Minicis, H. Uchinamiet al., Serum amyloid a induces inflammation, proliferation and cell death in activated hepatic stellate cells, Plos One, vol. 11, no. 3, p. e0150893, 2016. https://doi.org/10.1371/journal.pone.0150893
[3] Y. Wang, W. Wang, L. Wang, Y. Bao, J. Lu, Y. Lüet al., Extracellular matrix remodeling effects of serum amyloid a1 in the human amnion: implications for fetal membrane rupture, American Journal of Reproductive Immunology, vol. 81, no. 1, 2018. https://doi.org/10.1111/aji.13073
[4] X. Gan, W. Wang, J. Lu, L. Ling, Q. Zhou, H. Zhanget al., De novo synthesis of saa1 in the placenta participates in parturition, Frontiers in Immunology, vol. 11, 2020. https://doi.org/10.3389/fimmu.2020.01038
[5] Y. Wang, F. Cao, Y. Wang, G. Yu, & B. Jia, Silencing of saa1 inhibits palmitate- or high-fat diet induced insulin resistance through suppression of the nf-κb pathway, Molecular Medicine, vol. 25, no. 1, 2019. https://doi.org/10.1186/s10020-019-0075-4
[6] G. Li, Q. Shen, H. Xu, Y. Zhou, C. Li, Y. Liet al., Saa1 identified as a potential prediction biomarker for metastasis of hepatocellular carcinoma via multi-omics approaches, Frontiers in Oncology, vol. 13, 2023. https://doi.org/10.3389/fonc.2023.1138995
[7] L. Zhou, S. Duan, M. Zhou, M. Gu, S. Liu, Y. Wanget al., Mouse bone marrow mesenchymal stem cells inhibit sepsis-induced lung injury in mice via exosomal saa1, Molecular Pharmaceutics, vol. 19, no. 11, p. 4254-4263, 2022. https://doi.org/10.1021/acs.molpharmaceut.2c00542