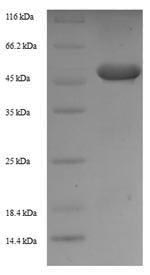
Recombinant Mouse Zinc finger protein 346 (Znf346) is produced in E.coli and includes the complete expression region spanning amino acids 1-294. The protein carries an N-terminal 6xHis-SUMO tag, which appears to improve both purification efficiency and overall stability. SDS-PAGE analysis confirms purity levels exceeding 90%, making it well-suited for detailed research applications. This product is designed exclusively for research purposes and should not be used for human or clinical applications.
Zinc finger protein 346 (Znf346) belongs to the zinc finger protein family, a group recognized for its DNA-binding capabilities and roles in transcription regulation. Znf346 participates in several cellular processes, including gene expression control and RNA binding, which may explain its growing importance in studies focused on gene regulation and cellular signaling networks. This protein seems particularly useful for researchers trying to understand how transcriptional control actually works.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse Znf346 is a zinc finger protein that requires precise folding around zinc ions and proper tertiary structure for its nucleic acid-binding function. The E. coli expression system can produce soluble proteins, and the SUMO tag may enhance solubility. However, zinc finger proteins often require specific chaperones and zinc availability for correct folding. While the full-length protein (1-294aa) contains all functional domains, the correct folding with functional nucleic acid-binding activity requires experimental validation.
1. Protein-Protein Interaction Studies Using Pull-Down Assays
Protein-protein interactions depend on native conformation. The full-length design supports interaction studies if proper folding is confirmed. If correctly folded, Znf346 could identify physiological interaction partners. However, misfolding may lead to non-specific binding or failure to recognize genuine partners. The SUMO tag may sterically hinder some interactions. Results require validation with an endogenous protein.
2. Antibody Development and Validation
This recombinant Znf346 serves as an excellent immunogen for generating antibodies against mouse Znf346. The full-length sequence ensures comprehensive epitope coverage. The His-SUMO tag facilitates purification and immunization procedures.
3. Biochemical Characterization and Stability Studies
This is the essential first step to assess protein quality. Techniques like size-exclusion chromatography can determine oligomeric state, while circular dichroism can analyze secondary structure and zinc-dependent folding. These studies provide critical quality control data about the protein's folding state and stability.
4. In Vitro Binding Assays with Nucleic Acids
Functional nucleic acid binding is entirely dependent on correct zinc finger domain folding. Nucleic acid binding requires precise tertiary structure formation around zinc ions. If properly folded, EMSA and SPR assays could reveal binding specificity. However, misfolding would yield false negatives or non-specific binding.
Final Recommendation & Action Plan
This recombinant Znf346 has potential for functional applications but requires rigorous folding validation before reliable use in interaction or binding studies. The immediate priority is Application 3 (Biochemical Characterization) to confirm proper folding through zinc supplementation assays and structural analysis. Application 2 (Antibody Development) can proceed immediately. Applications 1 and 4 should only be attempted after confirming correct folding through biophysical methods. For all functional applications, ensure adequate zinc availability in buffers to maintain structural integrity. This systematic approach ensures reliable interpretation of experimental outcomes.